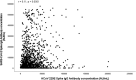
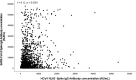
Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination?
- PMID: 36159791
- PMCID: PMC9493031
- DOI: 10.3389/fimmu.2022.954093
Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination?
Abstract
The SARS-CoV-2 belongs to the coronavirus family, which also includes common endemic coronaviruses (HCoVs). We hypothesized that immunity to HCoVs would be associated with stronger immunogenicity from SARS-CoV-2 vaccines. The study included samples from the COSRIP observational cohort study of adult paramedics in Canada. Participants provided blood samples, questionnaire data, and results of COVID-19 testing. Samples were tested for anti-spike IgG against SARS-CoV-2, HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43 antigens. We first compared samples from vaccinated and unvaccinated participants, to determine which HCoV antibodies were affected by vaccination. We created scatter plots and performed correlation analysis to estimate the extent of the linear relationship between HCoVs and SARS-CoV-2 anti-spike antibodies. Further, using adjusted log-log multiple regression, we modeled the association between each strain of HCoV and SARS-CoV-2 antibodies. Of 1510 participants (mean age of 39 years), 94 (6.2%) had a history of COVID-19. There were significant differences between vaccinated and unvaccinated participant in anti-spike antibodies to HCoV-HKU1, and HCoV-OC43; however, levels for HCoV-229E and HCoV-NL63 were similar (suggesting that vaccination did not affect these baseline values). Among vaccinated individuals without prior COVID-19 infection, SARS-COV-2 anti-spike IgG demonstrated a weak positive relationship between both HCoV-229E (r = 0.11) and HCoV-NL63 (r = 0.12). From the adjusted log-log multiple regression model, higher HCoV-229E and HCoV-NL63 anti-spike IgG antibodies were associated with increased SARS-COV-2 anti-spike IgG antibodies. Vaccination appears to result in measurable increases in HCoV-HKU1, and HCoV-OC43 IgG levels. Anti-HCoV-229E and HCoV-NL63 antibodies were unaffected by vaccination, and higher levels were associated with significantly higher COVID-19 vaccine-induced SARS-COV-2 antibodies.
Keywords: COVID-19; SARS-COV-2 antibodies; antibody concentrations; human endemic coronaviruses; vaccination.
Copyright © 2022 Asamoah-Boaheng, Grunau, Karim, Jassem, Bolster, Marquez, Scheuermeyer and Goldfarb.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination Boosts Neutralizing Activity Against Seasonal Human Coronaviruses.Clin Infect Dis. 2022 Aug 24;75(1):e653-e661. doi: 10.1093/cid/ciac057. Clin Infect Dis. 2022. PMID: 35079775 Free PMC article.
-
Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination.Front Cell Infect Microbiol. 2022 Sep 2;12:978440. doi: 10.3389/fcimb.2022.978440. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118022 Free PMC article.
-
Antibody Mediated Immunity to SARS-CoV-2 and Human Coronaviruses: Multiplex Beads Assay and Volumetric Absorptive Microsampling to Generate Immune Repertoire Cartography.Front Immunol. 2021 Jul 27;12:696370. doi: 10.3389/fimmu.2021.696370. eCollection 2021. Front Immunol. 2021. PMID: 34386006 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
Evolutionary trajectory of SARS-CoV-2 and emerging variants.Virol J. 2021 Aug 13;18(1):166. doi: 10.1186/s12985-021-01633-w. Virol J. 2021. PMID: 34389034 Free PMC article. Review.
Cited by
-
SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses.Vaccines (Basel). 2025 Mar 4;13(3):268. doi: 10.3390/vaccines13030268. Vaccines (Basel). 2025. PMID: 40266143 Free PMC article.
-
Immune responses of lung transplant recipients against SARS-CoV-2 and common respiratory coronaviruses: Evidence for pre-existing cross-reactive immunity.Transpl Immunol. 2023 Dec;81:101940. doi: 10.1016/j.trim.2023.101940. Epub 2023 Oct 20. Transpl Immunol. 2023. PMID: 37866672 Free PMC article.
-
Seasonal human coronavirus humoral responses in AZD1222 (ChaAdOx1 nCoV-19) COVID-19 vaccinated adults reveal limited cross-immunity.Front Immunol. 2024 May 17;15:1401728. doi: 10.3389/fimmu.2024.1401728. eCollection 2024. Front Immunol. 2024. PMID: 38827749 Free PMC article. Clinical Trial.
-
Longitudinal Surveillance of COVID-19 Antibodies in Pediatric Healthcare Workers.Vaccines (Basel). 2025 Feb 7;13(2):163. doi: 10.3390/vaccines13020163. Vaccines (Basel). 2025. PMID: 40006710 Free PMC article.
-
Seroprevalence and durability of antibody responses to AstraZeneca vaccination in Ugandans with prior mild or asymptomatic COVID-19: implications for vaccine policy.Front Immunol. 2023 May 2;14:1183983. doi: 10.3389/fimmu.2023.1183983. eCollection 2023. Front Immunol. 2023. PMID: 37205095 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) . Delta variant: What we know about the science (2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html.
-
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet (2021) 397(10280):1204–12. doi: 10.1016/S0140-6736(21)00575-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous