Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-κB/inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy
- PMID: 36159807
- PMCID: PMC9493454
- DOI: 10.3389/fimmu.2022.986593
Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-κB/inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy
Abstract
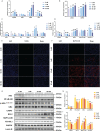
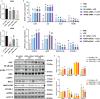
Vitamin D3 (VD3) participated widely in the nuclear factor-κB (NF-κB)-mediated inflammation, apoptosis, and autophagy through the vitamin D receptor (VDR). However, the molecular mechanisms remain not understood in teleost. The present study investigated the functions of VD3/VDR on intestinal inflammation, autophagy, and apoptosis of turbot in vivo and in vitro. Triple replicates of 30 fish were fed with each of three diets with graded levels of 32.0 (D0), 1012.6 (D1), and 3978.2 (D2) IU/kg VD3. Obvious intestinal enteritis was observed in the D0 group and followed with dysfunction of intestinal mucosal barriers. The intestinal inflammatory response induced by VD3 deficiency was regulated by the NF-κB/inflammasome signalling. The promotion of intestinal apoptosis and suppression of intestinal autophagy were also observed in the D0 group. Similarly, VD3 deficiency in vitro induced more intense inflammation regulated by NF-κB/inflammasome signalling. The mutually exclusive apoptosis and autophagy were also observed in the group without 1,25(OH)2D3 in vitro, accompanied by similar changes in apoptosis and autophagy increased apoptosis. The gene expression of VDRs was significantly increased with the increasing VD3 supplementation both in vivo and in vitro. Moreover, VDR knockdown in turbot resulted in intestinal inflammation, and this process relied on the activation of inflammasome mediated by NF-κB signalling. Simultaneously, intestinal apoptosis was promoted, whereas intestinal autophagy was inhibited. In conclusion, VD3 deficiency could induce intestinal inflammation via activation of the NF-κB/inflammasome pathway, intestinal apoptosis, and autophagy formed a mutually exclusive relation in teleost. And VDR is the critical molecule in those processes.
Keywords: NF-κB; apoptosis; autophagy; inflammasome; inflammation; vitamin D3; vitamin D3 receptor.
Copyright © 2022 Chen, Huang, Yongyut, Li, Esteban, Jintasataporn, Deng, Zhang, Ai, Mai and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SZ declared a shared affiliation with authors GL and JD to the editor at the time of review.
Figures

Similar articles
-
Vitamin D3/VDR inhibits inflammation through NF-κB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai.Cell Biol Toxicol. 2023 Jun;39(3):885-906. doi: 10.1007/s10565-021-09647-4. Epub 2021 Oct 12. Cell Biol Toxicol. 2023. PMID: 34637036
-
Vitamin D3 alleviates inflammation in ulcerative colitis by activating the VDR-NLRP6 signaling pathway.Front Immunol. 2023 Feb 8;14:1135930. doi: 10.3389/fimmu.2023.1135930. eCollection 2023. Front Immunol. 2023. PMID: 36845152 Free PMC article.
-
Vitamin D impacts on the intestinal health, immune status and metabolism in turbot (Scophthalmus maximus L.).Br J Nutr. 2022 Dec 14;128(11):2083-2096. doi: 10.1017/S0007114522000125. Epub 2022 Jan 21. Br J Nutr. 2022. PMID: 35057874
-
Vitamin D and systemic cancer: is this relevant to malignant melanoma?Br J Dermatol. 2002 Aug;147(2):197-213. doi: 10.1046/j.1365-2133.2002.04960.x. Br J Dermatol. 2002. PMID: 12174089 Review.
-
Cell cycle arrest and apoptosis induced by 1α,25(OH)2D3 and TX 527 in Kaposi sarcoma is VDR dependent.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:197-200. doi: 10.1016/j.jsbmb.2013.11.014. Epub 2013 Dec 5. J Steroid Biochem Mol Biol. 2014. PMID: 24316429 Review.
Cited by
-
Vitamin D as a Shield against Aging.Int J Mol Sci. 2023 Feb 25;24(5):4546. doi: 10.3390/ijms24054546. Int J Mol Sci. 2023. PMID: 36901976 Free PMC article. Review.
-
Vitamin D Promotes Mucosal Barrier System of Fish Skin Infected with Aeromonas hydrophila through Multiple Modulation of Physical and Immune Protective Capacity.Int J Mol Sci. 2023 Jul 8;24(14):11243. doi: 10.3390/ijms241411243. Int J Mol Sci. 2023. PMID: 37511003 Free PMC article.
-
Prevalence of Malnutrition and Micronutrient Deficiencies in Older Adults with Ulcerative Colitis.Dig Dis Sci. 2024 Nov;69(11):4203-4213. doi: 10.1007/s10620-024-08650-z. Epub 2024 Oct 22. Dig Dis Sci. 2024. PMID: 39438412
-
Dysregulation and gene polymorphisms of Vitamin D receptor: its implications in lipid metabolic disorders.Mol Biol Rep. 2025 Jun 23;52(1):630. doi: 10.1007/s11033-025-10725-7. Mol Biol Rep. 2025. PMID: 40549261 Review.
-
Modulation of the vitamin D/vitamin D receptor system in osteoporosis pathogenesis: insights and therapeutic approaches.J Orthop Surg Res. 2023 Nov 13;18(1):860. doi: 10.1186/s13018-023-04320-4. J Orthop Surg Res. 2023. PMID: 37957749 Free PMC article. Review.
References
-
- Chen Z, Liu Y, Li Y, Yang P, Hu H, Yu G, et al. . Dietary arginine supplementation mitigates the soybean meal induced enteropathy in juvenile turbot, Scophthalmus maximus L. Aquaculture Res (2018) 49:1535–45. doi: 10.1111/are.13608 - DOI
-
- Chen Z, Zhao S, Liu Y, Yang P, Ai Q, Zhang W, et al. . Dietary citric acid supplementation alleviates soybean meal-induced intestinal oxidative damage and micro-ecological imbalance in juvenile turbot, Scophthalmus maximus L. Aquaculture Res (2018) 49:3804–16. doi: 10.1111/are.13847 - DOI
-
- Liu S, Wang X, Bu X, Lin Z, Li E, Shi Q, et al. . Impact of dietary vitamin D3 supplementation on growth, molting, antioxidant capability, and immunity of juvenile Chinese mitten crabs (Eriocheir sinensis) by metabolites and vitamin D receptor. J Agric Food Chem (2021) 69:12794–806. doi: 10.1021/acs.jafc.1c04204 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

