LY6G6D is a selectively expressed colorectal cancer antigen that can be used for targeting a therapeutic T-cell response by a T-cell engager
- PMID: 36159851
- PMCID: PMC9493073
- DOI: 10.3389/fimmu.2022.1008764
LY6G6D is a selectively expressed colorectal cancer antigen that can be used for targeting a therapeutic T-cell response by a T-cell engager
Abstract
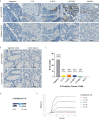
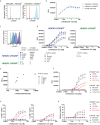
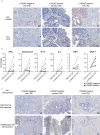
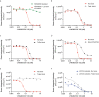
Colorectal cancer (CRC) is one of the most common cancers worldwide and demands more effective treatments. We sought to identify tumor selective CRC antigens and their therapeutic potential for cytotoxic T-cell targeting by transcriptomic and immunohistochemical analysis. LY6G6D was identified as a tumor selectively expressed CRC antigen, mainly in the microsatellite stable (MSS) subtype. A specific anti LY6G6D/CD3 T cell engager (TcE) was generated and demonstrated potent tumor cell killing and T cell activation in vitro. Ex vivo treatment of primary patient-derived CRC tumor slice cultures with the LY6G6D/CD3 TcE led to IFNγ secretion in LY6G6D positive tumor samples. In vivo, LY6G6D/CD3 TcE monotherapy demonstrated tumor regressions in pre-clinical mouse models of engrafted human CRC tumor cells and PBMCs. Lastly, 2D and 3D cocultures of LY6G6D positive and negative cells were used to explore the bystander killing of LY6G6D negative cells after specific activation of T cells by LY6G6D positive cells. LY6G6D/CD3 TcE treatment was shown to lyse target negative cells in the vicinity of target positive cells through a combined effect of IFNγ, TNFα and Fas/FasL. In summary, LY6G6D was identified as a selectively expressed CRC antigen that can be utilized to potently re-direct and activate cytotoxic T-cells to lyse LY6G6D expressing CRC using a TcE. This effect can be spread to target negative neighboring tumor cells, potentially leading to improved therapeutic efficacy.
Keywords: CD3; CRC (colorectal cancer); LY6G6D; TcE (T cell engager); immunotherapy.
Copyright © 2022 Corrales, Hipp, Martin, Sabarth, Tirapu, Fuchs, Thaler, Walterskirchen, Bauer, Fabits, Bergmann, Binder, Chetta, Vogt and Adam.
Conflict of interest statement
LC, SH, KM, NS, IT, KF, BT, CW, KB, PC, AV, and PA are employees of Boehringer Ingelheim affiliates. MB received research funding and speakers fee by Boehringer Ingelheim and Bristol Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

