Functional microvascularization of human myocardium in vitro
- PMID: 36160044
- PMCID: PMC9499876
- DOI: 10.1016/j.crmeth.2022.100280
Functional microvascularization of human myocardium in vitro
Abstract
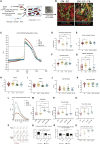
In this study, we report static and perfused models of human myocardial-microvascular interaction. In static culture, we observe distinct regulation of electrophysiology of human induced pluripotent stem cell derived-cardiomyocytes (hiPSC-CMs) in co-culture with human cardiac microvascular endothelial cells (hCMVECs) and human left ventricular fibroblasts (hLVFBs), including modification of beating rate, action potential, calcium handling, and pro-arrhythmic substrate. Within a heart-on-a-chip model, we subject this three-dimensional (3D) co-culture to microfluidic perfusion and vasculogenic growth factors to induce spontaneous assembly of perfusable myocardial microvasculature. Live imaging of red blood cells within myocardial microvasculature reveals pulsatile flow generated by beating hiPSC-CMs. This study therefore demonstrates a functionally vascularized in vitro model of human myocardium with widespread potential applications in basic and translational research.
Keywords: E-C coupling; cardiac physiology; cardiomyocyte; electrophysiology; endothelial cell; microphysiological systems; microvasculature; organ-on-chip; stem cell-derived models; tissue engineering.
© 2022 Imperial College London.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Beauchamp P., Jackson C.B., Ozhathil L.C., Agarkova I., Galindo C.L., Sawyer D.B., Suter T.M., Zuppinger C. 3D co-culture of hiPSC-derived cardiomyocytes with cardiac fibroblasts improves tissue-like features of cardiac spheroids. Front. Mol. Biosci. 2020;7:14. doi: 10.3389/fmolb.2020.00014. - DOI - PMC - PubMed
-
- Berry C., Duncker D.J. Coronary microvascular disease: the next frontier for cardiovascular research. Cardiovasc. Res. 2020;116:737–740. https://pubmed.ncbi.nlm.nih.gov/32149331/ - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

