Deep-learning analysis of micropattern-based organoids enables high-throughput drug screening of Huntington's disease models
- PMID: 36160045
- PMCID: PMC9500000
- DOI: 10.1016/j.crmeth.2022.100297
Deep-learning analysis of micropattern-based organoids enables high-throughput drug screening of Huntington's disease models
Abstract
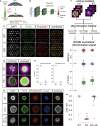
Organoids are carrying the promise of modeling complex disease phenotypes and serving as a powerful basis for unbiased drug screens, potentially offering a more efficient drug-discovery route. However, unsolved technical bottlenecks of reproducibility and scalability have prevented the use of current organoids for high-throughput screening. Here, we present a method that overcomes these limitations by using deep-learning-driven analysis for phenotypic drug screens based on highly standardized micropattern-based neural organoids. This allows us to distinguish between disease and wild-type phenotypes in complex tissues with extremely high accuracy as well as quantify two predictors of drug success: efficacy and adverse effects. We applied our approach to Huntington's disease (HD) and discovered that bromodomain inhibitors revert complex phenotypes induced by the HD mutation. This work demonstrates the power of combining machine learning with phenotypic drug screening and its successful application to reveal a potentially new druggable target for HD.
Keywords: Huntington's disease; bromodomains; deep learning; organoids.
© 2022 The Authors.
Conflict of interest statement
J.J.M., E.D.S., A.H.B., and F.E. are listed on a patent application regarding the screening approach; J.J.M. was a consultant for RUMI Scientific at the beginning of the project; A.H.B. and E.D.S are co-founders of RUMI Scientific; and A.H.B., E.D.S., and F.E. are shareholders of RUMI Scientific.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

