Unconventional genetic code systems in archaea
- PMID: 36160229
- PMCID: PMC9499178
- DOI: 10.3389/fmicb.2022.1007832
Unconventional genetic code systems in archaea
Abstract
Archaea constitute the third domain of life, distinct from bacteria and eukaryotes given their ability to tolerate extreme environments. To survive these harsh conditions, certain archaeal lineages possess unique genetic code systems to encode either selenocysteine or pyrrolysine, rare amino acids not found in all organisms. Furthermore, archaea utilize alternate tRNA-dependent pathways to biosynthesize and incorporate members of the 20 canonical amino acids. Recent discoveries of new archaeal species have revealed the co-occurrence of these genetic code systems within a single lineage. This review discusses the diverse genetic code systems of archaea, while detailing the associated biochemical elements and molecular mechanisms.
Keywords: archaea; genetic code expansion; phosphoserine; pyrrolysine; selenocysteine.
Copyright © 2022 Meng, Chung, Söll and Krahn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


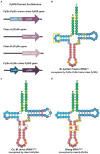
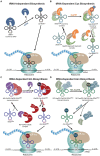
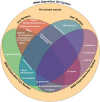
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

