First-Principles Insight into a B4C3 Monolayer as a Promising Biosensor for Exhaled Breath Analysis
- PMID: 36160759
- PMCID: PMC9484337
- DOI: 10.1007/s11664-022-09898-9
First-Principles Insight into a B4C3 Monolayer as a Promising Biosensor for Exhaled Breath Analysis
Abstract
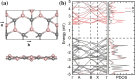
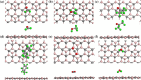
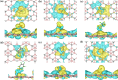
Nanomaterial-based room temperature gas sensors are used as a screening tool for diagnosing various diseases through breath analysis. The stable planar structure of boron carbide (B4C3) is utilized as a base material for adsorption of human breath exhaled VOCs, namely formaldehyde, methanol, acetone, toluene along, with interfering gases of carbon dioxide and water. The adsorption energy, charge density, density of states, energy band gap variation, recovery time, sensitivity, and work function of adsorbed molecules on pristine B4C3 are analyzed by density functional theory. The computed adsorption energies of VOC are in the range of - 0.176 to - 0.238 eV, and a larger interaction distance validate the physisorption behavior of these VOCs biomarkers on pristine boron carbide monolayer. Minute changes are determined from the electronic band structure of all adsorbed systems conserving the semiconducting nature of the B4C3 monolayer. The band gap variation upon adsorption of VOCs and interfering gases is examined between 0.05 and 0.52%. The 13.63 × 10-9 s recovery time of methanol is slower among VOCs, and 0.556 × 10-9 s of carbon dioxide (CO2) is faster for desorption. The results reveal that boron carbide can be utilized as a biosensor at room temperature for the analysis of exhaled VOCs from human breath.
Keywords: B4C3 monolayer; DFT study; Exhaled breath analysis; biosensor.
© The Minerals, Metals & Materials Society 2022.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures

References
-
- Chen H, et al. NiO–ZnO Nanoheterojunction Networks for Room-Temperature Volatile Organic Compounds Sensing. Adv. Opt. Mater. 2018;6:1. doi: 10.1002/adom.201800677. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
