Metabolomic and transcriptomic analyses reveal the mechanism of sweet-acidic taste formation during pineapple fruit development
- PMID: 36161024
- PMCID: PMC9493369
- DOI: 10.3389/fpls.2022.971506
Metabolomic and transcriptomic analyses reveal the mechanism of sweet-acidic taste formation during pineapple fruit development
Abstract
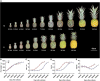
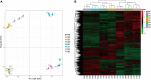
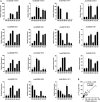
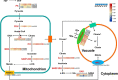
Pineapple (Ananas comosus L.) is one of the most valuable subtropical fruit crop in the world. The sweet-acidic taste of the pineapple fruits is a major contributor to the characteristic of fruit quality, but its formation mechanism remains elusive. Here, targeted metabolomic and transcriptomic analyses were performed during the fruit developmental stages in two pineapple cultivars ("Comte de Paris" and "MD-2") to gain a global view of the metabolism and transport pathways involved in sugar and organic acid accumulation. Assessment of the levels of different sugar and acid components during fruit development revealed that the predominant sugar and organic acid in mature fruits of both cultivars was sucrose and citric acid, respectively. Weighted gene coexpression network analysis of metabolic phenotypes and gene expression profiling enabled the identification of 21 genes associated with sucrose accumulation and 19 genes associated with citric acid accumulation. The coordinated interaction of the 21 genes correlated with sucrose irreversible hydrolysis, resynthesis, and transport could be responsible for sucrose accumulation in pineapple fruit. In addition, citric acid accumulation might be controlled by the coordinated interaction of the pyruvate-to-acetyl-CoA-to-citrate pathway, gamma-aminobutyric acid pathway, and tonoplast proton pumps in pineapple. These results provide deep insights into the metabolic regulation of sweetness and acidity in pineapple.
Keywords: Ananas comosus; citric acid; fruit quality; metabolic genes; sucrose; transporter genes.
Copyright © 2022 Gao, Yao, Chen, Wu, Wu, Liu, Guo and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Aslam M., Deng L., Wang X. B., Wang Y. (2019). Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci. Hortic. 257:108633. 10.1016/j.scienta.2019.108633 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

