COVID-19 vaccine-associated myocarditis
- PMID: 36161056
- PMCID: PMC9350606
- DOI: 10.4330/wjc.v14.i7.382
COVID-19 vaccine-associated myocarditis
Abstract
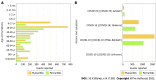
Myocarditis is now recognized as a rare complication of coronavirus disease 2019 (COVID-19) mRNA vaccination, particularly in adolescent and young adult males. Since the authorization of the Pfizer-BioNTech™ and Moderna™ mRNA vaccines targeting the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike protein, the Centers for Disease Control and Prevention (CDC) has reported 1175 confirmed cases of myocarditis after COVID-19 vaccination in individuals ages 30 years and younger as of January 2022. According to CDC data in June 2021, the incidence of vaccine-mediated myocarditis in males ages 12-29 years old was estimated to be 40.6 cases per million second doses of COVID-19 mRNA vaccination administered. Individuals with cases of COVID-19 vaccine-mediated myocarditis typically present with acute chest pain and elevated serum troponin levels, often within one week of receiving the second dose of mRNA COVID-19 vaccination. Most cases follow a benign clinical course with prompt resolution of symptoms. Proposed mechanisms of COVID-19 vaccine myocarditis include molecular mimicry between SARS-CoV-2 spike protein and self-antigens and the triggering of preexisting dysregulated immune pathways in predisposed individuals. The higher incidence of COVID-19 vaccine myocarditis in young males may be explained by testosterone and its role in modulating the immune response in myocarditis. There is limited data on long-term outcomes in these cases given the recency of their occurrence. The CDC continues to recommend COVID-19 vaccination for everyone 5 years of age and older given the greater risk of serious complications related to natural COVID-19 infection including hospitalization, multisystem organ dysfunction, and death. Further study is needed to better understand the immunopathology and long-term outcomes behind COVID-19 mRNA vaccine-mediated myocarditis.
Keywords: COVID-19; Myocarditis; Pericarditis; SARS-CoV-2; mRNA vaccine.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


References
-
- Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. - PMC - PubMed
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. - PMC - PubMed
-
- Centers for Disease Control and Prevention (CDC) COVID-19. Selected Adverse Events Reported after COVID-19 Vaccination. [cited 2 December 2021]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events... .
-
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

