Lovo-cel gene therapy for sickle cell disease: Treatment process evolution and outcomes in the initial groups of the HGB-206 study
- PMID: 36161320
- PMCID: PMC10092845
- DOI: 10.1002/ajh.26741
Lovo-cel gene therapy for sickle cell disease: Treatment process evolution and outcomes in the initial groups of the HGB-206 study
Abstract
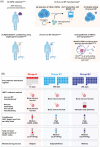
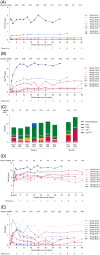
lovo-cel (bb1111; LentiGlobin for sickle cell disease [SCD]) gene therapy (GT) comprises autologous transplantation of hematopoietic stem and progenitor cells transduced with the BB305 lentiviral vector encoding a modified β-globin gene (βA-T87Q ) to produce anti-sickling hemoglobin (HbAT87Q ). The efficacy and safety of lovo-cel for SCD are being evaluated in the ongoing phase 1/2 HGB-206 study (ClinicalTrials.gov: NCT02140554). The treatment process evolved over time, using learnings from outcomes in the initial patients to optimize lovo-cel's benefit-risk profile. Following modest expression of HbAT87Q in the initial patients (Group A, n = 7), alterations were made to the treatment process for patients subsequently enrolled in Group B (n = 2, patients B1 and B2), including improvements to cell collection and lovo-cel manufacturing. After 6 months, median Group A peripheral blood vector copy number (≥0.08 c/dg) and HbAT87Q levels (≥0.46 g/dL) were inadequate for substantial clinical effect but stable and sustained over 5.5 years; both markedly improved in Group B (patient B1: ≥0.53 c/dg and ≥2.69 g/dL; patient B2: ≥2.14 c/dg and ≥6.40 g/dL, respectively) and generated improved biologic and clinical efficacy in Group B, including higher total hemoglobin and decreased hemolysis. The safety of the lovo-cel for SCD treatment regimen largely reflected the known side effects of HSPC collection, busulfan conditioning regimen, and underlying SCD; acute myeloid leukemia was observed in two patients in Group A and deemed unlikely related to insertional oncogenesis. Changes made during development of the lovo-cel treatment process were associated with improved outcomes and provide lessons for future SCD GT studies.
© 2022 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Julie Kanter reports honoraria from Medscape, Guidepoint Global, GLG, Jeffries, Cowen, and Wells Fargo; served as a consultant for bluebird bio, Novartis, Graphite, Fulcrum, and Axcella Rx; served on scientific advisory boards for bluebird bio, Novartis, Forma, and Beam; and reports membership of the National Alliance of Sickle Cell Disease and SCDAA Medical and Research Advisory Board.
Alexis A. Thompson reports research funding from CRISPR/Vertex, Novartis, Bristol Myers Squibb, Baxalta, BioMarin, and bluebird bio; and served as a consultant for Bristol Myers Squibb, Beam, Agios, and bluebird bio.
Francis J. Pierciey Jr. reports employment and equity ownership from bluebird bio.
Philippe Leboulch reports equity ownership from bluebird bio and patent ownership for the βA‐T87Q gene, the lovo‐cel BB305 lentiviral vector, and packaging plasmids.
Manfred Schmidt reports employment from the German Cancer Research Center and equity ownership from GeneWerk GmbH.
Melissa Bonner reports employment and equity ownership from bluebird bio.
Ruiting Guo reports employment and equity ownership from bluebird bio.
Alex Miller reports employment and equity ownership from bluebird bio.
Jean‐Antoine Ribeil reports equity ownership from bluebird bio.
David Davidson reports compensation and equity ownership from bluebird bio.
Mohammed Asmal reports equity ownership from bluebird bio.
Mark C. Walters served as medical director for AllCells and as a consultant for AllCells, Ensoma, Vertex, and BioLabs.
The remaining authors declare no competing financial interests.
Figures
References
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. - PubMed
-
- Bernaudin F, Socie G, Kuentz M, et al. Long‐term results of related myeloablative stem‐cell transplantation to cure sickle cell disease. Blood. 2007;110(7):2749‐2756. - PubMed
-
- Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea increases fetal hemoglobin production in sickle cell anemia. Trans Assoc Am Phys. 1984;97:268‐274. - PubMed
-
- Bradt PS, E., Synnott, P. , Chapman, R. , Rind, D.M. ; Pearson, S. Crizanlizumab, Voxelotor, and L‐glutamine for sickle cell disease: effectiveness and value. Institute for Clinical and Economic Review;2020.
