Integration of Universal Germline Genetic Testing for All New Breast Cancer Patients
- PMID: 36161375
- PMCID: PMC9512964
- DOI: 10.1245/s10434-022-12595-w
Integration of Universal Germline Genetic Testing for All New Breast Cancer Patients
Abstract
Background: The American Society of Breast Surgeons recommends genetic testing (GT) for all women with breast cancer (BC), but implementation and uptake of GT has not been well-described.
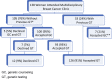
Methods: A retrospective chart review was performed for newly diagnosed BC patients or patients with a newly identified recurrence of BC seen in a multidisciplinary clinic (MDBC) who were offered genetic counseling (GC) and GT.
Results: The 138 women attending the MDBC had a median age of 54 years and comprised non-Hispanic whites (46%), Asians (28%), Hispanics (17%), blacks (4%), and other (5%). Of the 105 (76%) patients without prior GT, 100 (95%) accepted GC, with 93 (93%) of these 100 patients undergoing GT. The patients meeting the National Comprehensive Cancer Network (NCCN) guidelines for GT were more likely to undergo GT. Testing was performed with a 67- to 84-gene panel, together with an 8- to 9-gene STAT panel if needed. Among 120 patients with reports available, including 33 patients previously tested, 15 (12%) were positive (1 BLM, 1 BRCA1, 3 BRCA2, 1 BRIP1, 1 CFTR, 1 CHEK2, 1 MUTYH, 1 PALB2, 1 PRSS1, 1 RAD50, 1 RET, and 2 TP53), 44 (37%) were negative, and 61 (51%) had an uncertain variant. The median time to STAT results (n = 50) was 8 days. The STAT results were available before surgery for 47 (98%) of the 48 STAT patients undergoing surgery.
Conclusions: New BC patients attending the MDBC demonstrated high rates of acceptance of GC and GT. The combination of GC and GT can offer timely information critical to patient risk assessment and treatment planning.
© 2022. Society of Surgical Oncology.
Conflict of interest statement
Irene Kang has accepted consulting fees and speaker bureau fees for Puma Biotechnology and consulting fees for Bristol Myers Squibb. Ketan Patel received textbook royalties from Elsevier. The remaining authors have no conflicts of interest.
Figures

Comment in
-
ASO Author Reflections: A Green Light for Genetic Testing in All Patients with Breast Cancer.Ann Surg Oncol. 2023 Feb;30(2):1026-1027. doi: 10.1245/s10434-022-12671-1. Epub 2022 Oct 14. Ann Surg Oncol. 2023. PMID: 36241948 No abstract available.
References
-
- American College of Obstetricians and Gynecologists Hereditary cancer syndromes and risk assessment: ACOG committee opinion, no. 793. Obstet Gynecol. 2019;134:e143-9. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

