Access to Enantiomerically Pure P-Stereogenic Primary Aminophosphine Sulfides under Reductive Conditions
- PMID: 36161736
- PMCID: PMC10092265
- DOI: 10.1002/chem.202202608
Access to Enantiomerically Pure P-Stereogenic Primary Aminophosphine Sulfides under Reductive Conditions
Abstract
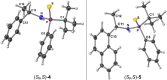
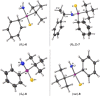
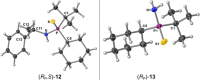
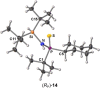
Stereochemically pure phosphines with phosphorus-heteroatom bonds and P-centered chirality are a promising class of functional building blocks for the design of chiral ligands and organocatalysts. A route to enantiomerically pure primary aminophosphine sulfides was opened through stereospecific reductive C-N bond cleavage of phosphorus(V) precursors by lithium in liquid ammonia. The chemoselectivity of the reaction as a function of reaction time, substrate pattern, and chiral auxiliary was investigated. In the presence of exclusively aliphatic groups bound to the phosphorus atom, all competing reductive side reactions are totally prevented. The absolute configurations of all P-stereogenic compounds were determined by single-crystal X-ray diffraction analysis. Their use as synthetic building blocks was demonstrated. The lithium salt of (R)-BINOL-dithiophosphoric acid proved to be a useful stereochemical probe to determine the enantiomeric purity. Insights into the coordination mode of the lithium-based chiral complex formed in solution was provided by NMR spectroscopy and DFT calculations.
Keywords: P-stereogenic compounds; alkali metals; cleavage reactions; phosphorus; structure elucidation.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




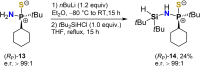
Similar articles
-
A Powerful P-N Connection: Preparative Approaches, Reactivity, and Applications of P-Stereogenic Aminophosphines.Chemistry. 2024 Mar 25;30(18):e202303760. doi: 10.1002/chem.202303760. Epub 2024 Jan 15. Chemistry. 2024. PMID: 38055219 Review.
-
P-Stereogenic Amino-Phosphines as Chiral Ligands: From Privileged Intermediates to Asymmetric Catalysis.Acc Chem Res. 2020 Mar 17;53(3):676-689. doi: 10.1021/acs.accounts.9b00633. Epub 2020 Feb 27. Acc Chem Res. 2020. PMID: 32105437
-
Application of P-stereogenic aminophosphine phosphinite ligands in asymmetric hydroformylation.Chemistry. 2000 Apr 14;6(8):1496-504. doi: 10.1002/(sici)1521-3765(20000417)6:8<1496::aid-chem1496>3.0.co;2-8. Chemistry. 2000. PMID: 10840972
-
Facile Synthesis of Enantiomerically Pure P-Chiral 1-Alkoxy-2,3-dihydrophospholes via Nucleophilic P-N Bond Cleavage of a 1-Phospha-2-azanorbornene.Chemistry. 2023 Aug 10;29(45):e202300790. doi: 10.1002/chem.202300790. Epub 2023 Jun 6. Chemistry. 2023. PMID: 37188645
-
P(III)-Chirogenic Phosphinite Building Blocks by Stereospecific N→O Phosphinyl Migration.J Org Chem. 2023 Dec 15;88(24):16679-16706. doi: 10.1021/acs.joc.3c01753. Epub 2023 Dec 2. J Org Chem. 2023. PMID: 38041618 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

