Potential new fluoroquinolone treatments for suspected bacterial keratitis
- PMID: 36161851
- PMCID: PMC9297210
- DOI: 10.1136/bmjophth-2022-001002
Potential new fluoroquinolone treatments for suspected bacterial keratitis
Abstract
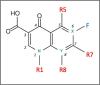
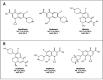
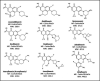
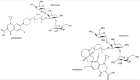
Topical fluoroquinolones (FQs) are an established treatment for suspected microbial keratitis. An increased FQ resistance in some classes of bacterial pathogens is a concern. Some recently developed FQs have an extended spectrum of activity, making them a suitable alternative for topical ophthalmic use. For example, the new generation FQs, avarofloxacin, delafloxacin, finafloxacin, lascufloxacin, nadifloxacin, levonadifloxacin, nemonoxacin and zabofloxacin have good activity against the common ophthalmic pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae and several of the Enterobacteriaceae However, because there are no published ophthalmic break-point concentrations, the susceptibility of an isolated micro-organism to a topical FQ is extrapolated from systemic break-point data and wild type susceptibility. The purpose of this review is to compare the pharmacokinetics and pharmacodynamics of the FQs licensed for topical ophthalmic use with the same parameters for new generation FQs. We performed a literature review of the FQs approved for topical treatment and the new generation FQs licensed to treat systemic infections. We then compared the minimum inhibitory concentrations (MIC) of bacterial isolates and the published concentrations that FQs achieved in the cornea and aqueous. We also considered the potential suitability of new generation FQs for topical use based on their medicinal properties. Notably, we found significant variation in the reported corneal and aqueous FQ concentrations so that reliance on the reported mean concentration may not be appropriate, and the first quartile concentration may be more clinically relevant. The provision of the MIC for the microorganism together with the achieved lower (first) quartile concentration of a FQ in the cornea could inform management decisions such as whether to continue with the prescribed antimicrobial, increase the frequency of application, use a combination of antimicrobials or change treatment.
Keywords: Cornea; Infection; Pharmacology; Treatment Medical.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Treatment of experimental bacterial keratitis with topical trovafloxacin.Arch Ophthalmol. 2004 Jan;122(1):65-9. doi: 10.1001/archopht.122.1.65. Arch Ophthalmol. 2004. PMID: 14718297
-
Using minimum inhibitory concentration values of common topical antibiotics to investigate emerging antibiotic resistance: A retrospective study of 134 dogs and 20 horses with ulcerative keratitis.Vet Ophthalmol. 2020 Sep;23(5):806-813. doi: 10.1111/vop.12801. Epub 2020 Jul 1. Vet Ophthalmol. 2020. PMID: 32608547
-
[In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,866 clinical isolates obtained from 72 centers in 2010].Jpn J Antibiot. 2012 Jun;65(3):181-206. Jpn J Antibiot. 2012. PMID: 23173294 Japanese.
-
A Literature-Based Review and Analysis of the Pharmacodynamics of the Dose Frequency of Topical 0.3% Ciprofloxacin and 0.3% Ofloxacin in the Day-1 Treatment of Bacterial Keratitis.J Ocul Pharmacol Ther. 2023 Jan-Feb;39(1):17-26. doi: 10.1089/jop.2022.0110. Epub 2022 Nov 29. J Ocul Pharmacol Ther. 2023. PMID: 36454630 Review.
-
Pharmacokinetic considerations in the treatment of bacterial keratitis.Clin Pharmacokinet. 1994 Aug;27(2):129-49. doi: 10.2165/00003088-199427020-00005. Clin Pharmacokinet. 1994. PMID: 7955776 Review.
Cited by
-
Mycobacterium chelonae Keratitis following Keratorefractive Lenticule Extraction: Highlighting Diagnostic and Treatment Complexities.Case Rep Ophthalmol. 2025 Mar 27;16(1):302-307. doi: 10.1159/000545563. eCollection 2025 Jan-Dec. Case Rep Ophthalmol. 2025. PMID: 40297754 Free PMC article.
-
Is there evidence for changes in antibiotic resistance of microorganisms causing postcataract surgery endophthalmitis: a systematic review.BMJ Open Ophthalmol. 2025 Apr 23;10(1):e001935. doi: 10.1136/bmjophth-2024-001935. BMJ Open Ophthalmol. 2025. PMID: 40274287 Free PMC article.
-
Activation of pro-resolving pathways mediate the therapeutic effects of thymosin beta-4 during Pseudomonas aeruginosa-induced keratitis.Front Immunol. 2024 Sep 24;15:1458684. doi: 10.3389/fimmu.2024.1458684. eCollection 2024. Front Immunol. 2024. PMID: 39380984 Free PMC article.
-
Increased tolerance to commonly used antibiotics in a Pseudomonas aeruginosa ex vivo porcine keratitis model.Microbiology (Reading). 2024 May;170(5):001459. doi: 10.1099/mic.0.001459. Microbiology (Reading). 2024. PMID: 38739119 Free PMC article.
-
Thymosin beta 4: A potential novel adjunct treatment for bacterial keratitis.Int Immunopharmacol. 2023 May;118:109953. doi: 10.1016/j.intimp.2023.109953. Epub 2023 Apr 3. Int Immunopharmacol. 2023. PMID: 37018981 Free PMC article.
References
-
- Stapleton F. The epidemiology of infectious keratitis. Ocul Surf (Published Online First: 19 August 2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
