A culture-free biphasic approach for sensitive and rapid detection of pathogens in dried whole-blood matrix
- PMID: 36161889
- PMCID: PMC9546527
- DOI: 10.1073/pnas.2209607119
A culture-free biphasic approach for sensitive and rapid detection of pathogens in dried whole-blood matrix
Erratum in
-
Correction for Ganguli et al., A culture-free biphasic approach for sensitive and rapid detection of pathogens in dried whole-blood matrix.Proc Natl Acad Sci U S A. 2025 Apr;122(13):e2504650122. doi: 10.1073/pnas.2504650122. Epub 2025 Mar 25. Proc Natl Acad Sci U S A. 2025. PMID: 40131957 Free PMC article. No abstract available.
Abstract
Blood stream infections (BSIs) cause high mortality, and their rapid detection remains a significant diagnostic challenge. Timely and informed administration of antibiotics can significantly improve patient outcomes. However, blood culture, which takes up to 5 d for a negative result, followed by PCR remains the gold standard in diagnosing BSI. Here, we introduce a new approach to blood-based diagnostics where large blood volumes can be rapidly dried, resulting in inactivation of the inhibitory components in blood. Further thermal treatments then generate a physical microscale and nanoscale fluidic network inside the dried matrix to allow access to target nucleic acid. The amplification enzymes and primers initiate the reaction within the dried blood matrix through these networks, precluding any need for conventional nucleic acid purification. High heme background is confined to the solid phase, while amplicons are enriched in the clear supernatant (liquid phase), giving fluorescence change comparable to purified DNA reactions. We demonstrate single-molecule sensitivity using a loop-mediated isothermal amplification reaction in our platform and detect a broad spectrum of pathogens, including gram-positive methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteria, gram-negative Escherichia coli bacteria, and Candida albicans (fungus) from whole blood with a limit of detection (LOD) of 1.2 colony-forming units (CFU)/mL from 0.8 to 1 mL of starting blood volume. We validated our assay using 63 clinical samples (100% sensitivity and specificity) and significantly reduced sample-to-result time from over 20 h to <2.5 h. The reduction in instrumentation complexity and costs compared to blood culture and alternate molecular diagnostic platforms can have broad applications in healthcare systems in developed world and resource-limited settings.
Keywords: biphasic; blood stream infection (BSI); isothermal amplification; porous dried blood matrix; sepsis diagnosis.
Conflict of interest statement
The authors declare no competing interest.
Figures

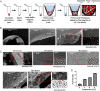
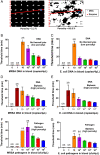
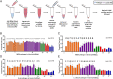

References
-
- Peker N., Couto N., Sinha B., Rossen J. W., Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: Recent developments in molecular approaches. Clin. Microbiol. Infect. 24, 944–955 (2018). - PubMed
-
- Novosad S. A., et al. , Vital signs: Epidemiology of sepsis: Prevalence of health care factors and opportunities for prevention. MMWR Morb. Mortal. Wkly. Rep. 65, 864–869 (2016). - PubMed
-
- Torio C. M., Andrews R. M., “Statistical brief #160 National inpatient hospital costs: The most expensive conditions by payer, 2011” in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs (Agency for Healthcare Research and Quality, 2013), vol. 31, pp. 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

