Single-molecule investigations of single-chain cellulose biosynthesis
- PMID: 36161928
- PMCID: PMC9546554
- DOI: 10.1073/pnas.2122770119
Single-molecule investigations of single-chain cellulose biosynthesis
Abstract
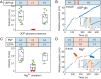
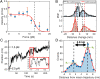
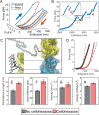
Cellulose biosynthesis in sessile bacterial colonies originates in the membrane-integrated bacterial cellulose synthase (Bcs) AB complex. We utilize optical tweezers to measure single-strand cellulose biosynthesis by BcsAB from Rhodobacter sphaeroides. Synthesis depends on uridine diphosphate glucose, Mg2+, and cyclic diguanosine monophosphate, with the last displaying a retention time of ∼80 min. Below a stall force of 12.7 pN, biosynthesis is relatively insensitive to force and proceeds at a rate of one glucose addition every 2.5 s at room temperature, increasing to two additions per second at 37°. At low forces, conformational hopping is observed. Single-strand cellulose stretching unveiled a persistence length of 6.2 nm, an axial stiffness of 40.7 pN, and an ability for complexes to maintain a tight grip, with forces nearing 100 pN. Stretching experiments exhibited hysteresis, suggesting that cellulose microstructure underpinning robust biofilms begins to form during synthesis. Cellohexaose spontaneously binds to nascent single cellulose strands, impacting polymer mechanical properties and increasing BcsAB activity.
Keywords: biosynthesis; cellulose; cellulose synthase; optical tweezers; single-molecule studies.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- O’Sullvian A. C., Cellulose: The structure slowly unravels. Cellulose 4, 173–207 (1997).
-
- McFarlane H. E., Döring A., Persson S., The cell biology of cellulose synthesis. Annu. Rev. Plant Biol. 65, 69–94 (2014). - PubMed
-
- Carroll A., Somerville C., Cellulosic biofuels. Annu. Rev. Plant Biol. 60, 165–182 (2009). - PubMed
-
- Otter J. A., et al. , Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 89, 16–27 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

