G protein-biased GPR3 signaling ameliorates amyloid pathology in a preclinical Alzheimer's disease mouse model
- PMID: 36161942
- PMCID: PMC9546571
- DOI: 10.1073/pnas.2204828119
G protein-biased GPR3 signaling ameliorates amyloid pathology in a preclinical Alzheimer's disease mouse model
Abstract
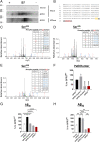
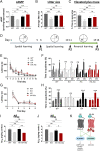
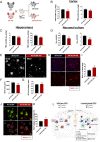
Biased G protein-coupled receptor (GPCR) ligands, which preferentially activate G protein or β-arrestin signaling pathways, are leading to the development of drugs with superior efficacy and reduced side effects in heart disease, pain management, and neuropsychiatric disorders. Although GPCRs are implicated in the pathophysiology of Alzheimer's disease (AD), biased GPCR signaling is a largely unexplored area of investigation in AD. Our previous work demonstrated that GPR3-mediated β-arrestin signaling modulates amyloid-β (Aβ) generation in vitro and that Gpr3 deficiency ameliorates Aβ pathology in vivo. However, Gpr3-deficient mice display several adverse phenotypes, including elevated anxiety-like behavior, reduced fertility, and memory impairment, which are potentially associated with impaired G protein signaling. Here, we generated a G protein-biased GPR3 mouse model to investigate the physiological and pathophysiological consequences of selective elimination of GPR3-mediated β-arrestin signaling in vivo. In contrast to Gpr3-deficient mice, G protein-biased GPR3 mice do not display elevated anxiety levels, reduced fertility, or cognitive impairment. We further determined that G protein-biased signaling reduces soluble Aβ levels and leads to a decrease in the area and compaction of amyloid plaques in the preclinical AppNL-G-F AD mouse model. The changes in amyloid pathology are accompanied by robust microglial and astrocytic hypertrophy, which suggest a protective glial response that may limit amyloid plaque development in G protein-biased GPR3 AD mice. Collectively, these studies indicate that GPR3-mediated G protein and β-arrestin signaling produce discrete and separable effects and provide proof of concept for the development of safer GPCR-targeting therapeutics with more directed pharmacological action for AD.
Keywords: Alzheimer’s disease; G protein–coupled receptor; amyloid plaques; arrestin; biased signaling.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

