Discovering design principles of collagen molecular stability using a genetic algorithm, deep learning, and experimental validation
- PMID: 36161946
- PMCID: PMC9546622
- DOI: 10.1073/pnas.2209524119
Discovering design principles of collagen molecular stability using a genetic algorithm, deep learning, and experimental validation
Abstract
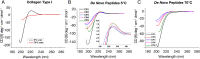
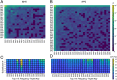
Collagen is the most abundant structural protein in humans, providing crucial mechanical properties, including high strength and toughness, in tissues. Collagen-based biomaterials are, therefore, used for tissue repair and regeneration. Utilizing collagen effectively during materials processing ex vivo and subsequent function in vivo requires stability over wide temperature ranges to avoid denaturation and loss of structure, measured as melting temperature (Tm). Although significant research has been conducted on understanding how collagen primary amino acid sequences correspond to Tm values, a robust framework to facilitate the design of collagen sequences with specific Tm remains a challenge. Here, we develop a general model using a genetic algorithm within a deep learning framework to design collagen sequences with specific Tm values. We report 1,000 de novo collagen sequences, and we show that we can efficiently use this model to generate collagen sequences and verify their Tm values using both experimental and computational methods. We find that the model accurately predicts Tm values within a few degrees centigrade. Further, using this model, we conduct a high-throughput study to identify the most frequently occurring collagen triplets that can be directly incorporated into collagen. We further discovered that the number of hydrogen bonds within collagen calculated with molecular dynamics (MD) is directly correlated to the experimental measurement of triple-helical quality. Ultimately, we see this work as a critical step to helping researchers develop collagen sequences with specific Tm values for intended materials manufacturing methods and biomedical applications, realizing a mechanistic materials by design paradigm.
Keywords: collagen; deep learning; generative algorithm; mechanics; thermal stability.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Sorushanova A., et al. , The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 31, e1801651 (2019). - PubMed
-
- Lodish H., Berk A., Zipursky S. L., Molecular Cell Biology: The Fibrous Proteins of the Matrix (W. H. Freeman, 2000).
-
- Prockop D. J., Kivirikko K. I., Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 (1995). - PubMed
-
- Ramachandran G. N., Kartha G., Structure of collagen. Nature 176, 593–595 (1955). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

