TFG regulates secretory and endosomal sorting pathways in neurons to promote their activity and maintenance
- PMID: 36161950
- PMCID: PMC9546632
- DOI: 10.1073/pnas.2210649119
TFG regulates secretory and endosomal sorting pathways in neurons to promote their activity and maintenance
Abstract
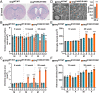
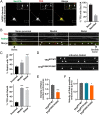
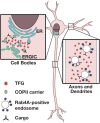
Molecular pathways that intrinsically regulate neuronal maintenance are poorly understood, but rare pathogenic mutations that underlie neurodegenerative disease can offer important insights into the mechanisms that facilitate lifelong neuronal function. Here, we leverage a rat model to demonstrate directly that the TFG p.R106C variant implicated previously in complicated forms of hereditary spastic paraplegia (HSP) underlies progressive spastic paraparesis with accompanying ventriculomegaly and thinning of the corpus callosum, consistent with disease phenotypes identified in adolescent patients. Analyses of primary cortical neurons obtained from CRISPR-Cas9-edited animals reveal a kinetic delay in biosynthetic secretory protein transport from the endoplasmic reticulum (ER), in agreement with prior induced pluripotent stem cell-based studies. Moreover, we identify an unexpected role for TFG in the trafficking of Rab4A-positive recycling endosomes specifically within axons and dendrites. Impaired TFG function compromises the transport of at least a subset of endosomal cargoes, which we show results in down-regulated inhibitory receptor signaling that may contribute to excitation-inhibition imbalances. In contrast, the morphology and trafficking of other organelles, including mitochondria and lysosomes, are unaffected by the TFG p.R106C mutation. Our findings demonstrate a multifaceted role for TFG in secretory and endosomal protein sorting that is unique to cells of the central nervous system and highlight the importance of these pathways to maintenance of corticospinal tract motor neurons.
Keywords: COPII; L1CAM; gephyrin; neurodegeneration.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Welniarz Q., Dusart I., Roze E., The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 77, 810–829 (2017). - PubMed
-
- Chaudhary R., Agarwal V., Rehman M., Kaushik A. S., Mishra V., Genetic architecture of motor neuron diseases. J. Neurol. Sci. 434, 120099 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

