Autocrine FGF1 signaling promotes glucose uptake in adipocytes
- PMID: 36161959
- PMCID: PMC9546606
- DOI: 10.1073/pnas.2122382119
Autocrine FGF1 signaling promotes glucose uptake in adipocytes
Abstract
Fibroblast growth factor 1 (FGF1) is an autocrine growth factor released from adipose tissue during over-nutrition or fasting to feeding transition. While local actions underlie the majority of FGF1's anti-diabetic functions, the molecular mechanisms downstream of adipose FGF receptor signaling are unclear. We investigated the effects of FGF1 on glucose uptake and its underlying mechanism in murine 3T3-L1 adipocytes and in ex vivo adipose explants from mice. FGF1 increased glucose uptake in 3T3-L1 adipocytes and epididymal WAT (eWAT) and inguinal WAT (iWAT). Conversely, glucose uptake was reduced in eWAT and iWAT of FGF1 knockout mice. We show that FGF1 acutely increased adipocyte glucose uptake via activation of the insulin-sensitive glucose transporter GLUT4, involving dynamic crosstalk between the MEK1/2 and Akt signaling proteins. Prolonged exposure to FGF1 stimulated adipocyte glucose uptake by MEK1/2-dependent transcription of the basal glucose transporter GLUT1. We have thus identified an alternative pathway to stimulate glucose uptake in adipocytes, independent from insulin, which could open new avenues for treating patients with type 2 diabetes.
Keywords: FGF1; adipocytes; fibroblast growth factors; glucose metabolism; insulin.
Conflict of interest statement
The authors declare no competing interest.
Figures
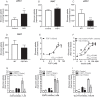
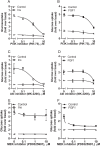

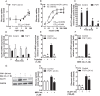
References
-
- Haslam D. W., James W. P. T., Obesity. Lancet 366, 1197–1209 (2005). - PubMed
-
- Tontonoz P., Spiegelman B. M., Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 (2008). - PubMed
-
- Mejhert N., et al. , Mapping of the fibroblast growth factors in human white adipose tissue. J. Clin. Endocrinol. Metab. 95, 2451–2457 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

