Intraligand Charge Transfer Enables Visible-Light-Mediated Nickel-Catalyzed Cross-Coupling Reactions
- PMID: 36161982
- PMCID: PMC9828175
- DOI: 10.1002/anie.202211433
Intraligand Charge Transfer Enables Visible-Light-Mediated Nickel-Catalyzed Cross-Coupling Reactions
Abstract
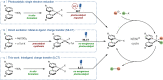
We demonstrate that several visible-light-mediated carbon-heteroatom cross-coupling reactions can be carried out using a photoactive NiII precatalyst that forms in situ from a nickel salt and a bipyridine ligand decorated with two carbazole groups (Ni(Czbpy)Cl2 ). The activation of this precatalyst towards cross-coupling reactions follows a hitherto undisclosed mechanism that is different from previously reported light-responsive nickel complexes that undergo metal-to-ligand charge transfer. Theoretical and spectroscopic investigations revealed that irradiation of Ni(Czbpy)Cl2 with visible light causes an initial intraligand charge transfer event that triggers productive catalysis. Ligand polymerization affords a porous, recyclable organic polymer for heterogeneous nickel catalysis of cross-coupling reactions. The heterogeneous catalyst shows stable performance in a packed-bed flow reactor during a week of continuous operation.
Keywords: Flow Chemistry; Heterogeneous Catalysis; Homogeneous Catalysis; Nickel Catalysis; Photocatalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Biffis A., Centomo P., Del Zotto A., Zecca M., Chem. Rev. 2018, 118, 2249–2295. - PubMed
-
- Ananikov V. P., ACS Catal. 2015, 5, 1964–1971.
-
- Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C., Nat. Chem. Rev. 2017, 1, 0052.
Grants and funding
- Liebig Fellowship/Verband der Chemischen Industrie
- Germany´s Excellence Strategy - EXC 2008 - 390540038 - UniSysCat/Deutsche Forschungsgemeinschaft
- BP 1635/2-19/Deutsche Forschungsgemeinschaft
- David and Lucile Packard Foundation
- DE-SC0018904/U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences
LinkOut - more resources
Full Text Sources

