Altered microbial biogeography in an innate model of colitis
- PMID: 36162004
- PMCID: PMC9519015
- DOI: 10.1080/19490976.2022.2123677
Altered microbial biogeography in an innate model of colitis
Abstract
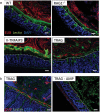
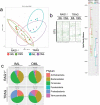
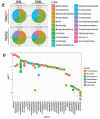
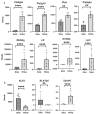
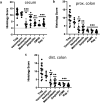
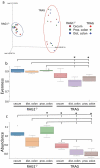
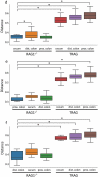
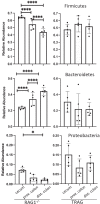
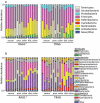
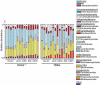
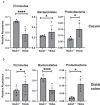
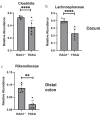
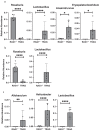
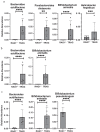
Changes in the spatial organization, or biogeography, of colonic microbes have been observed in human inflammatory bowel disease (IBD) and mouse models of IBD. We have developed a mouse model of IBD that occurs spontaneously and consistently in the absence of adaptive immunity. Mice expressing tumor necrosis factor-induced protein 3 (TNFAIP3) in intestinal epithelial cells (villin-TNFAIP3) develop colitis when interbred with Recombination Activating 1-deficient mice (RAG1<sup>-/-</sup>). The colitis in villin-TNFAIP3 × RAG1<sup>-/-</sup> (TRAG) mice is prevented by antibiotics, indicating a role for microbes in this innate colitis. We therefore explored the biogeography of microbes and responses to antibiotics in TRAG colitis. Laser capture microdissection and 16S rRNA sequencing revealed altered microbial populations across the transverse axis of the colon as the inner mucus layer of TRAG, but not RAG1<sup>-/-</sup>, mice was infiltrated by microbes, which included increased abundance of the classes Gammaproteobacteria and Actinobacteria. Along the longitudinal axis differences in the efficacy of antibiotics to prevent colitis were evident. Neomycin was most effective for prevention of inflammation in the cecum, while ampicillin was most effective in the proximal and distal colon. RAG1<sup>-/-</sup>, but not TRAG, mice exhibited a structured pattern of bacterial abundance with decreased Firmicutes and Proteobacteria but increased Bacteroidetes along the proximal to distal axis of the gut. TRAG mice exhibited increased relative abundance of potential pathobionts including <i>Bifidobacterium animalis</i> along the longitudinal axis of the gut whereas others, like <i>Helicobacter hepaticus</i> were increased only in the cecum. Potential beneficial organisms including <i>Roseburia</i> were decreased in the proximal regions of the TRAG colon, while <i>Bifidobacterium pseudolongulum</i> was decreased in the TRAG distal colon. Thus, the innate immune system maintains a structured, spatially organized, gut microbiome along the transverse and longitudinal axis of the gut, and disruption of this biogeography is a feature of innate immune colitis.
Keywords: A20; IBD; TRAG; biogeography; colitis; inflammatory bowel disease; innate immunity; laser capture microdissection; microbiome.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Overstreet AM, LaTorre DL, Abernathy-Close L, Murphy SF, Rhee L, Boger AM, Adlaka KR, Iverson AM, Bakke DS, Weber CR, et al. The JAK inhibitor ruxolitinib reduces inflammation in an ILC3-independent model of innate immune colitis. Mucosal Immunol. 2018;11(5):1454–24. doi:10.1038/s41385-018-0051-2. - DOI - PMC - PubMed
-
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi:10.1016/j.chom.2010.08.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
