Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Guideline Update
- PMID: 36162041
- PMCID: PMC9812449
- DOI: 10.1200/GO.22.00217
Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Guideline Update
Abstract
Purpose: To update resource-stratified, evidence-based recommendations on secondary prevention of cervical cancer globally.
Methods: American Society of Clinical Oncology convened a multidisciplinary, multinational Expert Panel to produce recommendations reflecting four resource-tiered settings. A review of existing guidelines, formal consensus-based process, and modified ADAPTE process to adapt existing guidelines was conducted. Other experts participated in formal consensus.
Results: This guideline update reflects changes in evidence since the previous update. Five existing guidelines were identified and reviewed, and adapted recommendations form the evidence base. Cost-effectiveness analyses provided indirect evidence to inform consensus, which resulted in ≥ 75% agreement.
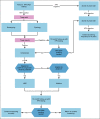
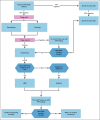
Recommendations: Human papillomavirus (HPV) DNA testing is recommended in all resource settings; visual inspection with acetic acid may be used in basic settings. Recommended age ranges and frequencies vary by the following setting: maximal: age 25-65 years, every 5 years; enhanced: age 30-65 years, if two consecutive negative tests at 5-year intervals, then every 10 years; limited: age 30-49 years, every 10 years; basic: age 30-49 years, one to three times per lifetime. For basic settings, visual assessment is used to determine treatment eligibility; in other settings, genotyping with cytology or cytology alone is used to determine treatment. For basic settings, treatment is recommended if abnormal triage results are obtained; in other settings, abnormal triage results followed by colposcopy is recommended. For basic settings, treatment options are thermal ablation or loop electrosurgical excision procedure; for other settings, loop electrosurgical excision procedure or ablation is recommended; with a 12-month follow-up in all settings. Women who are HIV-positive should be screened with HPV testing after diagnosis, twice as many times per lifetime as the general population. Screening is recommended at 6 weeks postpartum in basic settings; in other settings, screening is recommended at 6 months. In basic settings without mass screening, infrastructure for HPV testing, diagnosis, and treatment should be developed.Additional information is available at www.asco.org/resource-stratified-guidelines.
Conflict of interest statement
Figures



References
-
- James SE, Herman JL, Rankin S: The Report of the 2015 U.S. Transgender Survey. Washington, DC, National Center for Transgender Equality, 2016

