Neoadjuvant Radiation Therapy and Surgery Improves Metastasis-Free Survival over Surgery Alone in a Primary Mouse Model of Soft Tissue Sarcoma
- PMID: 36162051
- PMCID: PMC9812921
- DOI: 10.1158/1535-7163.MCT-21-0991
Neoadjuvant Radiation Therapy and Surgery Improves Metastasis-Free Survival over Surgery Alone in a Primary Mouse Model of Soft Tissue Sarcoma
Abstract
This study aims to investigate whether adding neoadjuvant radiotherapy (RT), anti-programmed cell death protein-1 (PD-1) antibody (anti-PD-1), or RT + anti-PD-1 to surgical resection improves disease-free survival for mice with soft tissue sarcomas (STS). We generated a high mutational load primary mouse model of STS by intramuscular injection of adenovirus expressing Cas9 and guide RNA targeting Trp53 and intramuscular injection of 3-methylcholanthrene (MCA) into the gastrocnemius muscle of wild-type mice (p53/MCA model). We randomized tumor-bearing mice to receive isotype control or anti-PD-1 antibody with or without radiotherapy (20 Gy), followed by hind limb amputation. We used micro-CT to detect lung metastases with high spatial resolution, which was confirmed by histology. We investigated whether sarcoma metastasis was regulated by immunosurveillance by lymphocytes or tumor cell-intrinsic mechanisms. Compared with surgery with isotype control antibody, the combination of anti-PD-1, radiotherapy, and surgery improved local recurrence-free survival (P = 0.035) and disease-free survival (P = 0.005), but not metastasis-free survival. Mice treated with radiotherapy, but not anti-PD-1, showed significantly improved local recurrence-free survival and metastasis-free survival over surgery alone (P = 0.043 and P = 0.007, respectively). The overall metastasis rate was low (∼12%) in the p53/MCA sarcoma model, which limited the power to detect further improvement in metastasis-free survival with addition of anti-PD-1 therapy. Tail vein injections of sarcoma cells into immunocompetent mice suggested that impaired metastasis was due to inability of sarcoma cells to grow in the lungs rather than a consequence of immunosurveillance. In conclusion, neoadjuvant radiotherapy improves metastasis-free survival after surgery in a primary model of STS.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures
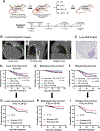
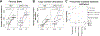

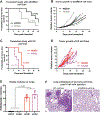
References
-
- Wang D, Zhang Q, Eisenberg BL, Kane JM, Li XA, Lucas D, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(20):2231–8 doi 10.1200/JCO.2014.58.5828. - DOI - PMC - PubMed
-
- Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res 2003;9(6):1941–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

