Systemic LSD1 Inhibition Prevents Aberrant Remodeling of Metabolism in Obesity
- PMID: 36162056
- PMCID: PMC9750949
- DOI: 10.2337/db21-1131
Systemic LSD1 Inhibition Prevents Aberrant Remodeling of Metabolism in Obesity
Abstract
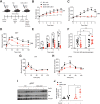
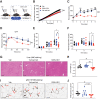
The transition from lean to obese states involves systemic metabolic remodeling that impacts insulin sensitivity, lipid partitioning, inflammation, and glycemic control. Here, we have taken a pharmacological approach to test the role of a nutrient-regulated chromatin modifier, lysine-specific demethylase (LSD1), in obesity-associated metabolic reprogramming. We show that systemic administration of an LSD1 inhibitor (GSK-LSD1) reduces food intake and body weight, ameliorates nonalcoholic fatty liver disease (NAFLD), and improves insulin sensitivity and glycemic control in mouse models of obesity. GSK-LSD1 has little effect on systemic metabolism of lean mice, suggesting that LSD1 has a context-dependent role in promoting maladaptive changes in obesity. In analysis of insulin target tissues we identified white adipose tissue as the major site of insulin sensitization by GSK-LSD1, where it reduces adipocyte inflammation and lipolysis. We demonstrate that GSK-LSD1 reverses NAFLD in a non-hepatocyte-autonomous manner, suggesting an indirect mechanism potentially via inhibition of adipocyte lipolysis and subsequent effects on lipid partitioning. Pair-feeding experiments further revealed that effects of GSK-LSD1 on hyperglycemia and NAFLD are not a consequence of reduced food intake and weight loss. These findings suggest that targeting LSD1 could be a strategy for treatment of obesity and its associated complications including type 2 diabetes and NAFLD.
© 2022 by the American Diabetes Association.
Figures





References
-
- Sarma S, Sockalingam S, Dash S. Obesity as a multisystem disease: trends in obesity rates and obesity-related complications. Diabetes Obes Metab 2021;23(Suppl. 1):3–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

