The journey of a lifetime - development of Pfizer's COVID-19 vaccine
- PMID: 36162187
- PMCID: PMC9433349
- DOI: 10.1016/j.copbio.2022.102803
The journey of a lifetime - development of Pfizer's COVID-19 vaccine
Abstract
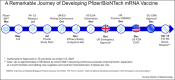
It would be apt to say that one of the greatest accomplishments in modern medicine has been the development of vaccines against COVID-19, which had paralyzed the entire world for more than a year. Pfizer and BioNTech codeveloped the first COVID-19 vaccine that was granted emergency-use authorization or conditional approval in several regions globally. This article is an attempt to go 'behind-the-scenes' of this development process and highlight key factors that allowed us to move with this unprecedented speed, while adhering to normal vaccine-development requirements to generate the information the regulatory authorities needed to assess the safety and effectiveness of a vaccine to prevent an infectious disease, including quality and manufacturing standards. This is also a story of how Pfizer and BioNTech leveraged our combined skill sets and experience to respond to the global health crisis to progress this program swiftly while ensuring the compliance with our high-quality standards and keeping patient safety at the forefront. We will also highlight multiple other factors that were instrumental in our success.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures




References
-
- Pollard C., De Koker S., Saelens X., Vanham G., Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol Med. 2013;19:705–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

