Subcortical brain volumes in young infants exposed to antenatal maternal depression: Findings from a South African birth cohort
- PMID: 36162238
- PMCID: PMC9668606
- DOI: 10.1016/j.nicl.2022.103206
Subcortical brain volumes in young infants exposed to antenatal maternal depression: Findings from a South African birth cohort
Abstract
Background: Several studies have reported enlarged amygdala and smaller hippocampus volumes in children and adolescents exposed to maternal depression. It is unclear whether similar volumetric differences are detectable in the infants' first weeks of life, following exposure in utero. We investigated subcortical volumes in 2-to-6 week old infants exposed to antenatal maternal depression (AMD) from a South African birth cohort.
Methods: AMD was measured with the Beck Depression Inventory 2nd edition (BDI-II) at 28-32 weeks gestation. T2-weighted structural images were acquired during natural sleep on a 3T Siemens Allegra scanner. Subcortical regions were segmented based on the University of North Carolina neonatal brain atlas. Volumetric estimates were compared between AMD-exposed (BDI-II ⩾ 20) and unexposed (BDI-II < 14) infants, adjusted for age, sex and total intracranial volume using analysis of covariance.
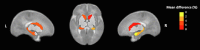
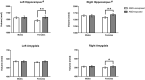
Results: Larger volumes were observed in AMD-exposed (N = 49) compared to unexposed infants (N = 75) for the right amygdala (1.93% difference, p = 0.039) and bilateral caudate nucleus (left: 5.79% difference, p = 0.001; right: 6.09% difference, p < 0.001). A significant AMD-by-sex interaction was found for the hippocampus (left: F(1,118) = 4.80, p = 0.030; right: F(1,118) = 5.16, p = 0.025), reflecting greater volume in AMD-exposed females (left: 5.09% difference, p = 0.001, right: 3.54% difference, p = 0.010), but not males.
Conclusions: Volumetric differences in subcortical regions can be detected in AMD-exposed infants soon after birth, suggesting structural changes may occur in utero. Female infants might exhibit volumetric changes that are not observed in male infants. The potential mechanisms underlying these early volumetric differences, and their significance for long-term child mental health, require further investigation.
Keywords: Brain morphometry; Child development; Depressive disorders; Magnetic resonance imaging; Prenatal stress; Sex differences.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Early structural brain development in infants exposed to HIV and antiretroviral therapy in utero in a South African birth cohort.J Int AIDS Soc. 2022 Jan;25(1):e25863. doi: 10.1002/jia2.25863. J Int AIDS Soc. 2022. PMID: 35041774 Free PMC article.
-
Antenatal maternal intimate partner violence exposure is associated with sex-specific alterations in brain structure among young infants: Evidence from a South African birth cohort.Dev Cogn Neurosci. 2023 Apr;60:101210. doi: 10.1016/j.dcn.2023.101210. Epub 2023 Feb 6. Dev Cogn Neurosci. 2023. PMID: 36764039 Free PMC article.
-
Alcohol exposure in utero is associated with decreased gray matter volume in neonates.Metab Brain Dis. 2016 Feb;31(1):81-91. doi: 10.1007/s11011-015-9771-0. Epub 2015 Nov 29. Metab Brain Dis. 2016. PMID: 26616173 Free PMC article.
-
Association of Prenatal Exposure to Early-Life Adversity With Neonatal Brain Volumes at Birth.JAMA Netw Open. 2022 Apr 1;5(4):e227045. doi: 10.1001/jamanetworkopen.2022.7045. JAMA Netw Open. 2022. PMID: 35412624 Free PMC article.
-
Windows of Opportunity: How Age and Sex Shape the Influence of Prenatal Depression on the Child Brain.Biol Psychiatry. 2025 Feb 1;97(3):227-247. doi: 10.1016/j.biopsych.2024.07.022. Epub 2024 Aug 6. Biol Psychiatry. 2025. PMID: 39117167 Review.
Cited by
-
Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy.Mol Psychiatry. 2024 Jul;29(7):2223-2240. doi: 10.1038/s41380-024-02449-0. Epub 2024 Feb 28. Mol Psychiatry. 2024. PMID: 38418579 Free PMC article. Review.
-
Prenatal exposure to extreme ambient heat may amplify the adverse impact of Superstorm Sandy on basal ganglia volume among school-aged children.PLoS One. 2025 Jun 11;20(6):e0324150. doi: 10.1371/journal.pone.0324150. eCollection 2025. PLoS One. 2025. PMID: 40498678 Free PMC article.
-
Prenatal stress alters mouse offspring dorsal striatal development and placental function in sex-specific ways.J Psychiatr Res. 2025 Feb;182:149-160. doi: 10.1016/j.jpsychires.2024.12.048. Epub 2025 Jan 1. J Psychiatr Res. 2025. PMID: 39809011
-
Antenatal Opioid Exposure and Global and Regional Brain Volumes in Newborns.JAMA Pediatr. 2025 Jun 1;179(6):639-646. doi: 10.1001/jamapediatrics.2025.0277. JAMA Pediatr. 2025. PMID: 40193106
-
Maternal perinatal depression and child brain structure at 2-3 years in a South African birth cohort study.Transl Psychiatry. 2023 Mar 20;13(1):96. doi: 10.1038/s41398-023-02395-5. Transl Psychiatry. 2023. PMID: 36941258 Free PMC article.
References
-
- Acosta H., Tuulari J.J., Scheinin N.M., Hashempour N., Rajasilta O., Lavonius T.I., Pelto J., Saunavaara V., Parkkola R., Lahdesmaki T., Karlsson L., Karlsson H. Prenatal maternal depressive symptoms are associated with smaller amygdalar volumes of four-year-old children. Psychiatry Research-Neuroimaging. 2020;304 - PubMed
-
- Acosta H., Kantojarvi K., Hashempour N., Pelto J., Scheinin N.M., Lehtola S.J., Lewis J.D., Fonov V.S., Collins D.L., Evans A., Parkkola R., Lahdesmaki T., Saunavaara J., Karlsson L., Merisaari H., Paunio T., Karlsson H., Tuulari J.J. Partial support for an interaction between a polygenic risk score for major depressive disorder and prenatal maternal depressive symptoms on infant right amygdalar volumes. Cereb. Cortex. 2020;30:6121–6134. - PubMed
-
- Acosta H., Kantojarvi K., Tuulari J.J., Lewis J.D., Hashempour N., Scheinin N.M., Lehtola S.J., Fonov V.S., Collins D.L., Evans A., Parkkola R., Lahdesmaki T., Saunavaara J., Merisaari H., Karlsson L., Paunio T., Karlsson H. Sex-specific association between infant caudate volumes and a polygenic risk score for major depressive disorder. J. Neurosci. Res. 2020;98:2529–2540. - PubMed
-
- Adamson B., Letourneau N., Lebel C. Prenatal maternal anxiety and children's brain structure and function: A systematic review of neuroimaging studies. J. Affect. Disord. 2018;241:117–126. - PubMed
-
- Andersen S. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

