LCK inhibition downregulates YAP activity and is therapeutic in patient-derived models of cholangiocarcinoma
- PMID: 36162702
- PMCID: PMC11410293
- DOI: 10.1016/j.jhep.2022.09.014
LCK inhibition downregulates YAP activity and is therapeutic in patient-derived models of cholangiocarcinoma
Abstract
Background & aims: There is an unmet need to develop novel, effective medical therapies for cholangiocarcinoma (CCA). The Hippo pathway effector, Yes-associated protein (YAP), is oncogenic in CCA, but has historically been difficult to target therapeutically. Recently, we described a novel role for the LCK proto-oncogene, Src family tyrosine kinase (LCK) in activating YAP through tyrosine phosphorylation. This led to the hypothesis that LCK is a viable therapeutic target in CCA via regulation of YAP activity.
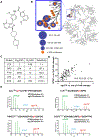
Methods: A novel tyrosine kinase inhibitor with relative selectivity for LCK, NTRC 0652-0, was pharmacodynamically profiled in vitro and in CCA cells. A panel of eight CCA patient-derived organoids were characterized and tested for sensitivity to NTRC 0652-0. Two patient-derived xenograft models bearing fibroblast growth factor receptor 2 (FGFR2)-rearrangements were utilized for in vivo assessment of pharmacokinetics, toxicity, and efficacy.
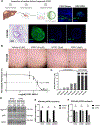
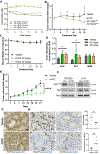
Results: NTRC 0652-0 demonstrated selectivity for LCK inhibition in vitro and in CCA cells. LCK inhibition with NTRC 0652-0 led to decreased tyrosine phosphorylation, nuclear localization, and co-transcriptional activity of YAP, and resulted in apoptotic cell death in CCA cell lines. A subset of tested patient-derived organoids demonstrated sensitivity to NTRC 0652-0. CCAs with FGFR2 fusions were identified as a potentially susceptible and clinically relevant genetic subset. In patient-derived xenograft models of FGFR2 fusion-positive CCA, daily oral treatment with NTRC 0652-0 resulted in stable plasma and tumor drug levels, acceptable toxicity, decreased YAP tyrosine phosphorylation, and significantly decreased tumor growth.
Conclusions: A novel LCK inhibitor, NTRC 0652-0, inhibited YAP signaling and demonstrated preclinical efficacy in CCA cell lines, and patient-derived organoid and xenograft models.
Impact and implications: Although aberrant YAP activation is frequently seen in CCA, YAP targeted therapies are not yet clinically available. Herein we show that a novel LCK-selective tyrosine kinase inhibitor (NTRC 0652-0) effectively inhibits YAP tyrosine phosphorylation and cotranscriptional activity and is well tolerated and cytotoxic in multiple preclinical models. The data suggest this approach may be effective in CCA with YAP dependence or FGFR2 fusions, and these findings warrant further investigation in phase I clinical trials.
Keywords: Hippo pathway; Src family kinase; bile duct tumors; tyrosine kinase inhibitor.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Competing interests: R.C. Buijsman is managing director and shareholder of Netherlands Translational Research Center B.V. Other authors declare no potential conflicts of interest.
Figures

References
-
- Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic Analysis of FGFR2-Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov 2021;11:326–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

