PFKFB3-mediated Pro-glycolytic Shift in Hepatocellular Carcinoma Proliferation
- PMID: 36162723
- PMCID: PMC9672450
- DOI: 10.1016/j.jcmgh.2022.09.009
PFKFB3-mediated Pro-glycolytic Shift in Hepatocellular Carcinoma Proliferation
Abstract
Background & aims: Metabolic reprogramming, in particular, glycolytic regulation, supports abnormal survival and growth of hepatocellular carcinoma (HCC) and could serve as a therapeutic target. In this study, we sought to identify glycolytic regulators in HCC that could be inhibited to prevent tumor progression and could also be monitored in vivo, with the goal of providing a theragnostic alternative to existing therapies.
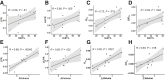
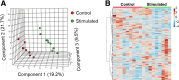
Methods: An orthotopic HCC rat model was used. Tumors were stimulated into a high-proliferation state by use of off-target liver ablation and were compared with lower-proliferating controls. We measured in vivo metabolic alteration in tumors before and after stimulation, and between stimulated tumors and control tumors using hyperpolarized 13C magnetic resonance imaging (MRI) (h13C MRI). We compared metabolic alterations detected by h13C MRI to metabolite levels from ex vivo mass spectrometry, mRNA levels of key glycolytic regulators, and histopathology.
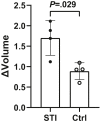
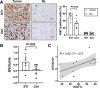
Results: Glycolytic lactate flux increased within HCC tumors 3 days after tumor stimulation, correlating positively with tumor proliferation as measured with Ki67. This was associated with a shift towards aerobic glycolysis and downregulation of the pentose phosphate pathway detected by mass spectrometry. MRI-measured lactate flux was most closely coupled with PFKFB3 expression and was suppressed with direct inhibition using PFK15.
Conclusions: Inhibition of PFKFB3 prevents glycolytic-mediated HCC proliferation, trackable by in vivo h13C MRI.
Keywords: Aerobic Glycolysis; Hyperpolarized (13)C MRI.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Llovet J.M., Villanueva A., Lachenmayer A., Finn R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. - PubMed
-
- Amann T., Maegdefrau U., Hartmann A., Agaimy A., Marienhagen J., Weiss T.S., Stoeltzing O., Warnecke C., Schölmerich J., Oefner P.J., Kreutz M., Bosserhoff A.K., Hellerbrand C. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. - PMC - PubMed
-
- Calvisi D.F., Wang C., Ho C., Ladu S., Lee S.A., Mattu S., Destefanis G., Delogu S., Zimmermann A., Ericsson J., Brozzetti S., Staniscia T., Chen X., Dombrowski F., Evert M. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. - PMC - PubMed
-
- Suzuki H., Kohjima M., Tanaka M., Goya T., Itoh S., Yoshizumi T., Mori M., Tsuda M., Takahashi M., Kurokawa M., Imoto K., Tashiro S., Kuwano A., Kato M., Okada S., Nakamuta M., Ogawa Y. Metabolic alteration in hepatocellular carcinoma: mechanism of lipid accumulation in well-differentiated hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2021;2021 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

