Characterization of IMG Microglial Cell Line as a Valuable In Vitro Tool for NLRP3 Inflammasome Studies
- PMID: 36163404
- PMCID: PMC11412188
- DOI: 10.1007/s10571-022-01285-6
Characterization of IMG Microglial Cell Line as a Valuable In Vitro Tool for NLRP3 Inflammasome Studies
Abstract
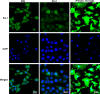
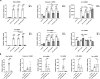
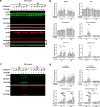
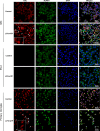
Microglial cells constantly surveil the cerebral microenvironment and become activated following injury and disease to mediate inflammatory responses. The nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3 (NLRP3) inflammasome, which is abundantly expressed in microglial cells, plays a key role in these responses as well as in the development of many neurological disorders. Microglial cell lines are a valuable tool to study the causes and possible treatments for neurological diseases which are linked to inflammation. Here, we investigated whether the mouse microglial cell line IMG is suitable to study NLRP3 inflammasome by incubating cells with different concentrations of NLRP3 inflammasome priming and activating agents lipopolysaccharide (LPS) and ATP, respectively, and applying short (4 h) or long (24 h) LPS incubation times. After short LPS incubation, the mRNA levels of most pro-inflammatory and NLRP3 inflammasome-associated genes were more upregulated than after long incubation. Moreover, the combination of higher LPS and ATP concentrations with short incubation time resulted in greater levels of active forms of caspase-1 and interleukin-1 beta (IL-1β) proteins than low LPS and ATP concentrations or long incubation time. We also demonstrated that treatment with NLRP3 inflammasome inhibitor glibenclamide suppressed NLRP3 inflammasome activation in IMG cells, as illustrated by the downregulation of gasdermin D N-fragment and mature caspase-1 and IL-1β protein levels. In addition, we conducted similar experiments with primary microglial cells and BV-2 cell line to determine the similarities and differences in their responses. Overall, our results indicate that IMG cell line could be a valuable tool for NLRP3 inflammasome studies. In IMG cells, 4-h incubation with lipopolysaccharide (LPS) induces a stronger upregulation of NLRP3 inflammasome-associated pro-inflammatory genes compared to 24-h incubation. NLRP3 inflammasome is robustly activated only after the addition of 3 mM of ATP following short LPS incubation time.
Keywords: IMG cells; Microglia; NLRP3 inflammasome; Neuroinflammation.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


Similar articles
-
Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia.Neurosci Lett. 2020 Sep 25;736:135279. doi: 10.1016/j.neulet.2020.135279. Epub 2020 Jul 26. Neurosci Lett. 2020. PMID: 32726591
-
Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway.Front Immunol. 2019 Jul 2;10:1511. doi: 10.3389/fimmu.2019.01511. eCollection 2019. Front Immunol. 2019. PMID: 31327964 Free PMC article.
-
Estradiol inhibits NLRP3 inflammasome in fibroblast-like synoviocytes activated by lipopolysaccharide and adenosine triphosphate.Int J Rheum Dis. 2018 Nov;21(11):2002-2010. doi: 10.1111/1756-185X.13198. Epub 2017 Nov 3. Int J Rheum Dis. 2018. PMID: 29105345
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Targeting NLRP3 Inflammasomes: A Trojan Horse Strategy for Intervention in Neurological Disorders.Mol Neurobiol. 2025 Feb;62(2):1840-1881. doi: 10.1007/s12035-024-04359-2. Epub 2024 Jul 23. Mol Neurobiol. 2025. PMID: 39042218 Review.
Cited by
-
Incretin-Based Multi-Agonist Peptides Are Neuroprotective and Anti-Inflammatory in Cellular Models of Neurodegeneration.Biomolecules. 2024 Jul 19;14(7):872. doi: 10.3390/biom14070872. Biomolecules. 2024. PMID: 39062586 Free PMC article.
References
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183(2):787–791. 10.4049/jimmunol.0901363 - PMC - PubMed
-
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27(2–3):229–237. 10.1016/0165-5728(90)90073-v - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. 10.1006/abio.1976.9999 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

