Haplotype-aware analysis of somatic copy number variations from single-cell transcriptomes
- PMID: 36163550
- PMCID: PMC10289836
- DOI: 10.1038/s41587-022-01468-y
Haplotype-aware analysis of somatic copy number variations from single-cell transcriptomes
Abstract
Genome instability and aberrant alterations of transcriptional programs both play important roles in cancer. Single-cell RNA sequencing (scRNA-seq) has the potential to investigate both genetic and nongenetic sources of tumor heterogeneity in a single assay. Here we present a computational method, Numbat, that integrates haplotype information obtained from population-based phasing with allele and expression signals to enhance detection of copy number variations from scRNA-seq. Numbat exploits the evolutionary relationships between subclones to iteratively infer single-cell copy number profiles and tumor clonal phylogeny. Analysis of 22 tumor samples, including multiple myeloma, gastric, breast and thyroid cancers, shows that Numbat can reconstruct the tumor copy number profile and precisely identify malignant cells in the tumor microenvironment. We identify genetic subpopulations with transcriptional signatures relevant to tumor progression and therapy resistance. Numbat requires neither sample-matched DNA data nor a priori genotyping, and is applicable to a wide range of experimental settings and cancer types.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures
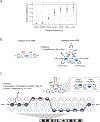


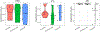
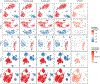
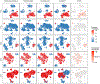








References
Methods-only References
-
- Huang X & Huang Y Cellsnp-lite: an efficient tool for genotyping single cells. Bioinformatics 37, 4569–4571 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

