A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering
- PMID: 36163606
- PMCID: PMC9512960
- DOI: 10.1007/s12033-022-00567-0
A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering
Abstract
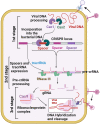
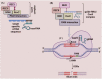
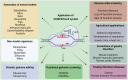
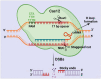
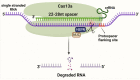
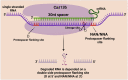
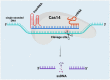
The clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (CRISPR/Cas) system has altered life science research offering enormous options in manipulating, detecting, imaging, and annotating specific DNA or RNA sequences of diverse organisms. This system incorporates fragments of foreign DNA (spacers) into CRISPR cassettes, which are further transcribed into the CRISPR arrays and then processed to make guide RNA (gRNA). The CRISPR arrays are genes that encode Cas proteins. Cas proteins provide the enzymatic machinery required for acquiring new spacers targeting invading elements. Due to programmable sequence specificity, numerous Cas proteins such as Cas9, Cas12, Cas13, and Cas14 have been exploited to develop new tools for genome engineering. Cas variants stimulated genetic research and propelled the CRISPR/Cas tool for manipulating and editing nucleic acid sequences of living cells of diverse organisms. This review aims to provide detail on two classes (class 1 and 2) of the CRISPR/Cas system, and the mechanisms of all Cas proteins, including Cas12, Cas13, and Cas14 discovered so far. In addition, we also discuss the pros and cons and recent applications of various Cas proteins in diverse fields, including those used to detect viruses like severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This review enables the researcher to gain knowledge on various Cas proteins and their applications, which have the potential to be used in next-generation precise genome engineering.
Keywords: CRISPR; Cas system; DETECTR; Genome engineering; SHERLOCK.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

