Noncoding RNAs Associated with PPARs in Etiology of MAFLD as a Novel Approach for Therapeutics Targets
- PMID: 36164476
- PMCID: PMC9509273
- DOI: 10.1155/2022/6161694
Noncoding RNAs Associated with PPARs in Etiology of MAFLD as a Novel Approach for Therapeutics Targets
Abstract
Background: Metabolic associated fatty liver disease (MAFLD) is a complex disease that results from the accumulation of fat in the liver. MAFLD is directly associated with obesity, insulin resistance, diabetes, and metabolic syndrome. PPARγ ligands, including pioglitazone, are also used in the management of this disease. Noncoding RNAs play a critical role in various diseases such as diabetes, obesity, and liver diseases including MAFLD. However, there is no adequate knowledge about the translation of using these ncRNAs to the clinics, particularly in MAFLD conditions. The aim of this study was to identify ncRNAs in the etiology of MAFLD as a novel approach to the therapeutic targets.
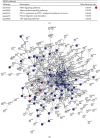
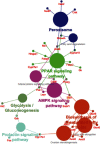
Methods: We collected human and mouse MAFLD gene expression datasets available in GEO. We performed pathway enrichment analysis of total mRNAs based on KEGG repository data to screen the most potential pathways in the liver of MAFLD human subjects and mice model, and analyzed pathway interconnections via ClueGO. Finally, we screened disease causality of the MAFLD ncRNAs, which were associated with PPARs, and then discussed the role of revealed ncRNAs in PPAR signaling and MAFLD.
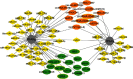
Results: We found 127 ncRNAs in MAFLD which 25 out of them were strongly validated before for regulation of PPARs. With a polypharmacology approach, we screened 51 ncRNAs which were causal to a subset of diseases related to MAFLD.
Conclusion: This study revealed a subset of ncRNAs that could help in more clear and guided designation of preclinical and clinical studies to verify the therapeutic application of the revealed ncRNAs by manipulating the PPARs molecular mechanism in MAFLD.
Copyright © 2022 Fatemeh Kazeminasab et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures



References
LinkOut - more resources
Full Text Sources

