Efficacy of COVID-19 Convalescent Plasma Based on Antibody Concentration
- PMID: 36164495
- PMCID: PMC9509285
- DOI: 10.1155/2022/7992927
Efficacy of COVID-19 Convalescent Plasma Based on Antibody Concentration
Abstract
Background: Convalescent plasma obtained from individuals who have recovered from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contains neutralizing antibodies to the virus and has been frequently used as a treatment in hospitalized patients with severe COVID-19.
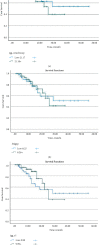
Methods: We conducted a retrospective, observational cohort study involving 96 hospitalized patients with severe COVID-19 who were allocated in a 1 : 1 ratio to having received either high antibody concentration convalescent plasma or low antibody concentration convalescent plasma. Quantitative measurements of IgG to the receptor-binding domain (RBD), the S1 subunit of the spike protein, and the SARS-CoV-2 nucleocapsid (N) protein were determined from donor plasma samples. The primary outcome was all-cause mortality within 30 days following convalescent plasma administration in regard to each of the three antibody domains.
Results: Within the nucleocapsid antibody domain, death occurred in 22.2% of patients in the low antibody concentration group versus 23.5% in the high antibody concentration group (p=0.88). Within the RBD antibody domain, death occurred in 22.9% of patients in both the low and the high antibody concentration groups (p=1.0). Within the S1 subunit antibody domain, death occurred in 27.1% of patients in the low antibody concentration group versus 18.8% in the high antibody concentration group (p=0.33).
Conclusions: No significant differences were observed between low and high concentration convalescent plasma in regard to overall mortality at 30 days, hospital length of stay, number of ventilator days, and subsequent receipt of invasive mechanical ventilation in patients who were previously not receiving mechanical ventilation. Trial Registration. This study was not associated with a clinical trial due to the retrospective nature of study design.
Copyright © 2022 Wesley V Cain et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- COVID-19 Map . Johns Hopkins Coronavirus Resource Center; 2021. https://coronavirus.jhu.edu/map.html .
LinkOut - more resources
Full Text Sources
Miscellaneous

