Expert Panel Consensus for Addressing Anti-VEGF Treatment Challenges of Diabetic Macular Edema in Spain
- PMID: 36164581
- PMCID: PMC9507974
- DOI: 10.2147/OPTH.S374763
Expert Panel Consensus for Addressing Anti-VEGF Treatment Challenges of Diabetic Macular Edema in Spain
Abstract
Purpose: The treatment of diabetic macular edema (DME) has evolved rapidly in the past decade, highlighting the need to address the challenges of routine clinical practice decision-making through expert consensus agreements.
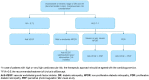
Methods: After a literature review and discussion of real-world experience on DME management, a group of ten retina specialists agreed on a consensus of recommendations for the most appropriate management of DME patients using vascular endothelial growth factor inhibitors (anti-VEGF) in Spain.
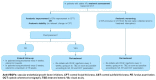
Results: The panel recommended early treatment initiation in DME patients with worse baseline visual acuity (VA) to maintain or improve outcome. For patients with good VA, an observation strategy was recommended, considering the presence of diabetic retinopathy, optical coherence tomography biomarkers, and impact on patient's quality of life. Based on the available evidence and clinical experience, the panel recommended the use of anti-VEGF intensive loading doses with the objective of achieving anatomic and visual responses as soon as possible, followed by a Treat & Extend (T&E) strategy to maintain VA improvement. Aflibercept was recommended for patients with a baseline decimal VA <0.5, followed by a T&E strategy, including the possibility to extend frequency of injections up to 16 weeks.
Conclusion: An expert panel proposes a consensus for the management of DME in Spain. Early treatment initiation with anti-VEGF in DME patients is recommended to maintain or improve VA; aflibercept is recommended for patients with a poor baseline VA.
Keywords: clinical practice patterns; consensus; diabetic macular edema; intravitreal injections.
© 2022 Fernández-Vigo et al.
Conflict of interest statement
José Ignacio Fernández-Vigo has received honoraria for advisory boards, lectures, and travel costs from Bayer, Novartis, Roche and Allergan. Inés Contreras and Rosario Cobo have received honoraria for advisory boards, lectures, and travel costs from Bayer, Novartis, and Allergan. María José Crespo and Luis Arrevola-Velasco have received honoraria for advisory boards, lectures and travel costs from Bayer. Ignacio Flores-Moreno has received honoraria for advisory boards from Bayer and Novartis. Jesús Pareja has received honoraria for advisory boards, lectures and travel costs from Bayer and Allergan, and María Dolores Martín has received honoraria for lectures from Bayer and Pfizer. Dr Carlos Beckford reports non-financial support from Novartis, non-financial support from Allergan, non-financial support from Bayer, during the conduct of the study; non-financial support from Novartis, non-financial support from Allergan, non-financial support from Bayer, outside the submitted work. The rest of the authors received honoraria for starting the advisory board from Bayer. The authors report no other conflicts of interest in this work.
Figures
References
-
- Factsheets | IDF Diabetes Atlas. Available from: https://diabetesatlas.org/regional-factsheets/. Accessed November 30, 2021.
-
- International Diabetes Federation. Clinical Practice Recommendations for Managing Diabetic Macular Edema. 2019th. International Diabetes Federation; 2019.
-
- Romero-Aroca P, Fernández-Alart J, Baget-Bernaldiz M, Méndez-Marín I, Salvat-Serra M. [Diabetic retinopathy epidemiology in type II diabetic patients. Effect of the changes in the diagnostic criteria and stricter control of the diabetes between 1993 and 2005 on the incidence of diabetic retinopathy]. Arch Soc Esp Oftalmol. 2007;82(4):209–218. Spanish. doi:10.4321/S0365-66912007000400005 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous