Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis
- PMID: 36164984
- PMCID: PMC9588706
- DOI: 10.1161/CIRCRESAHA.122.320184
Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis
Abstract
Background: Despite available clinical management strategies, chronic kidney disease (CKD) is associated with severe morbidity and mortality worldwide, which beckons new solutions. Host-microbial interactions with a depletion of Faecalibacterium prausnitzii in CKD are reported. However, the mechanisms about if and how F prausnitzii can be used as a probiotic to treat CKD remains unknown.
Methods: We evaluated the microbial compositions in 2 independent CKD populations for any potential probiotic. Next, we investigated if supplementation of such probiotic in a mouse CKD model can restore gut-renal homeostasis as monitored by its effects on suppression on renal inflammation, improvement in gut permeability and renal function. Last, we investigated the molecular mechanisms underlying the probiotic-induced beneficial outcomes.
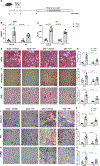
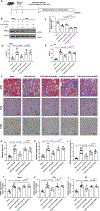
Results: We observed significant depletion of Faecalibacterium in the patients with CKD in both Western (n=283) and Eastern populations (n=75). Supplementation of F prausnitzii to CKD mice reduced renal dysfunction, renal inflammation, and lowered the serum levels of various uremic toxins. These are coupled with improved gut microbial ecology and intestinal integrity. Moreover, we demonstrated that the beneficial effects in kidney induced by F prausnitzii-derived butyrate were through the GPR (G protein-coupled receptor)-43.
Conclusions: Using a mouse CKD model, we uncovered a novel beneficial role of F prausnitzii in the restoration of renal function in CKD, which is, at least in part, attributed to the butyrate-mediated GPR-43 signaling in the kidney. Our study provides the necessary foundation to harness the therapeutic potential of F prausnitzii for ameliorating CKD.
Keywords: Faecalibacterium; butyrates; kidney diseases; microbiota; receptors, G-protein-coupled.
Figures
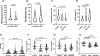


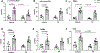


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

