Population pharmacokinetic-pharmacodynamic analyses of amyloid positron emission tomography and plasma biomarkers for lecanemab in subjects with early Alzheimer's disease
- PMID: 36165093
- PMCID: PMC9755918
- DOI: 10.1002/psp4.12862
Population pharmacokinetic-pharmacodynamic analyses of amyloid positron emission tomography and plasma biomarkers for lecanemab in subjects with early Alzheimer's disease
Abstract
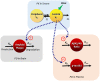
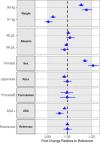
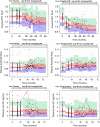
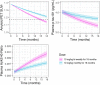
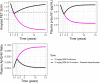
Lecanemab is a humanized immunoglobulin G1 monoclonal antibody that selectively binds to soluble Aβ aggregate species, while demonstrating low affinity for Aβ monomer. This article describes the population pharmacokinetic (PK) and PK/pharmacodynamic (PD) analyses for amyloid plaques, as measured using positron emission tomography (PET), and biomarkers of amyloid pathology as evidenced by Aβ42/40 ratio and plasma p-tau181 following i.v. administration of lecanemab in subjects with early Alzheimer's disease. Lecanemab PKs were well-characterized with a two-compartment model with first-order elimination. Final PK model contained covariate effects of anti-drug antibody positive status, sex, body weight, and albumin on clearance. The time course of amyloid PET standard uptake ratio (SUVr), plasma Aβ42/40 ratio, and p-tau181 were described using indirect response models with lecanemab exposure as a maximum effect function stimulating the reduction of SUVr, and as a linear function increasing Aβ42/40 ratio and decreasing p-tau181 formation rates. PK/PD simulations show that 10 mg/kg biweekly dosing results in larger and faster decrease in SUVr and p-tau181 and increase in Aβ42/40 ratio as compared to 10 mg/kg monthly dose. Furthermore, the PK/PD simulations showed that after treatment discontinuation the brain amyloid re-accumulation to baseline levels is slow with a recovery half-life of ~4 years, whereas plasma Aβ42/40 ratio and p-tau181 return to baseline levels faster than amyloid. Given the relationship between changes in amyloid PET SUVr and soluble biomarkers, the developed PK/PD models can be used to inform lecanemab dose regimens in future clinical studies.
© 2022 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
S.H., O.T., A.K., and S.Y. are employees of Eisai Co., Ltd. S.R., I.L., C.S., and L.R. are employees of Eisai Inc. Z.H. is an employee of Eisai Ltd.
Figures
References
-
- Alzheimer's Association . Alzheimer's disease facts and figures; 2021. Available from: https://www.alz.org/media/Documents/alzheimers‐facts‐and‐figures.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

