Immunodeficient patient experience of emergency switch from intravenous to rapid push subcutaneous immunoglobulin replacement therapy during coronavirus disease 2019 shielding
- PMID: 36165464
- PMCID: PMC9612677
- DOI: 10.1097/ACI.0000000000000864
Immunodeficient patient experience of emergency switch from intravenous to rapid push subcutaneous immunoglobulin replacement therapy during coronavirus disease 2019 shielding
Abstract
Purpose of review: Welsh immunodeficient patients on immunoglobulin replacement therapy (IgRT) who were considered high risk for severe coronavirus disease 2019 (COVID-19) were directed to shield. Consequently, patients receiving hospital-based intravenous immunoglobulin (IVIg) quickly transitioned to home-based self-administered subcutaneous immunoglobulin (SCIg). This evaluation aimed to assess patients' perceptions and experiences and laboratory outcomes of emergency IgRT transition during COVID-19.
Recent findings: A quick transition from in-hospital IVIg to home-based rapid push SCIg is achievable, however, patient IgRT administration preference remains key outside of emergency shielding measures.
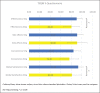
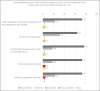
Summary: Subjective self-reported experiences ( n = 23) and objective immunoglobulin G (IgG) concentration ( n = 28) assessments were prospectively collected from patients pre/post-IgRT switch. In total, 41/55 (75%) patients transitioned from IVIg to rapid push SCIg and all completed training to self-administer subcutaneously within 24 days. Twenty-two percent ( n = 5) of patients preferred SCIg and 35% ( n = 8) wanted to return to hospital-based IVIg at 6 weeks post-transition. Mean IgG levels were similar pre vs. post-SCIg switch (10.3 g/l vs. 10.6 g/l, respectively). Patients reported greater infection anxiety during COVID-19 and adapted behaviours to mitigate risk. Although a third of patients wished to return to IVIg following cessation of shielding, over time the percentage electing to remain on SCIg rose from 22% to 59%.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures

References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. - PubMed
-
- Public Health England. Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. 2020. Available at: https://www.gov.uk/government/publications/guidance-on-shielding-and-pro....
-
- Welsh Government. Guidance on protecting people defined on medical grounds as clinically extremely vulnerable from coronavirus (COVID-19) – previously known as ‘shielding’. 2020. Available at: https://gov.wales/guidance-on-shielding-and-protecting-people-defined-on....
-
- Freedman L. Strategy for a pandemic: the UK and COVID-19. Survival 2020; 62:25–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

