HfaE Is a Component of the Holdfast Anchor Complex That Tethers the Holdfast Adhesin to the Cell Envelope
- PMID: 36165621
- PMCID: PMC9664946
- DOI: 10.1128/jb.00273-22
HfaE Is a Component of the Holdfast Anchor Complex That Tethers the Holdfast Adhesin to the Cell Envelope
Abstract
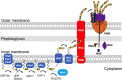
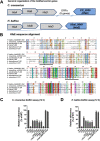
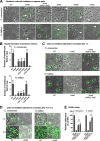
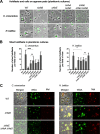
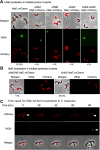
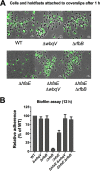
Bacteria use adhesins to colonize different surfaces and form biofilms. The species of the Caulobacterales order use a polar adhesin called holdfast, composed of polysaccharides, proteins, and DNA, to irreversibly adhere to surfaces. In Caulobacter crescentus, a freshwater Caulobacterales species, the holdfast is anchored at the cell pole via the
Keywords: Caulobacterales; adhesion; bacterial adhesin; biofilm; biofilms; extracellular polysaccharides; holdfast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
What Glues the Glue to the Cell Surface?J Bacteriol. 2022 Nov 15;204(11):e0038622. doi: 10.1128/jb.00386-22. Epub 2022 Oct 26. J Bacteriol. 2022. PMID: 36286485 Free PMC article.
Similar articles
-
Mutations in Sugar-Nucleotide Synthesis Genes Restore Holdfast Polysaccharide Anchoring to Caulobacter crescentus Holdfast Anchor Mutants.J Bacteriol. 2018 Jan 10;200(3):e00597-17. doi: 10.1128/JB.00597-17. Print 2018 Feb 1. J Bacteriol. 2018. PMID: 29158242 Free PMC article.
-
A Multiprotein Complex Anchors Adhesive Holdfast at the Outer Membrane of Caulobacter crescentus.J Bacteriol. 2019 Aug 22;201(18):e00112-19. doi: 10.1128/JB.00112-19. Print 2019 Sep 15. J Bacteriol. 2019. PMID: 31061167 Free PMC article.
-
A localized multimeric anchor attaches the Caulobacter holdfast to the cell pole.Mol Microbiol. 2010 Apr;76(2):409-27. doi: 10.1111/j.1365-2958.2010.07106.x. Epub 2010 Mar 10. Mol Microbiol. 2010. PMID: 20233308 Free PMC article.
-
RTX Adhesins are Key Bacterial Surface Megaproteins in the Formation of Biofilms.Trends Microbiol. 2019 May;27(5):453-467. doi: 10.1016/j.tim.2018.12.003. Epub 2019 Jan 15. Trends Microbiol. 2019. PMID: 30658900 Review.
-
Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria.Microbiol Spectr. 2015 Aug;3(4):10.1128/microbiolspec.MB-0018-2015. doi: 10.1128/microbiolspec.MB-0018-2015. Microbiol Spectr. 2015. PMID: 26350310 Free PMC article. Review.
Cited by
-
What Glues the Glue to the Cell Surface?J Bacteriol. 2022 Nov 15;204(11):e0038622. doi: 10.1128/jb.00386-22. Epub 2022 Oct 26. J Bacteriol. 2022. PMID: 36286485 Free PMC article.
-
Bacteria in Fluid Flow.J Bacteriol. 2023 Apr 25;205(4):e0040022. doi: 10.1128/jb.00400-22. Epub 2023 Mar 23. J Bacteriol. 2023. PMID: 36951552 Free PMC article. Review.
References
-
- Lee CK, Vachier J, de Anda J, Zhao K, Baker AE, Bennett RR, Armbruster CR, Lewis KA, Tarnopol RL, Lomba CJ, Hogan DA, Parsek MR, O’Toole GA, Golestanian R, Wong GCL. 2020. Social cooperativity of bacteria during reversible surface attachment in young biofilms: a quantitative comparison of Pseudomonas aeruginosa PA14 and PAO1. mBio 11:e02644-19. 10.1128/mBio.02644-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

