Blue Light Signaling Regulates Escherichia coli W1688 Biofilm Formation and l-Threonine Production
- PMID: 36165805
- PMCID: PMC9604211
- DOI: 10.1128/spectrum.02460-22
Blue Light Signaling Regulates Escherichia coli W1688 Biofilm Formation and l-Threonine Production
Abstract
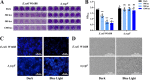
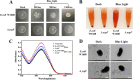
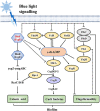
Escherichia coli biofilm may form naturally on biotic and abiotic surfaces; this represents a promising approach for efficient biochemical production in industrial fermentation. Recently, industrial exploitation of the advantages of optogenetics, such as simple operation, high spatiotemporal control, and programmability, for regulation of biofilm formation has garnered considerable attention. In this study, we used the blue light signaling-induced optogenetic system Magnet in an E. coli biofilm-based immobilized fermentation system to produce l-threonine in sufficient quantity. Blue light signaling significantly affected the phenotype of E. coli W1688. A series of biofilm-related experiments confirmed the inhibitory effect of blue light signaling on E. coli W1688 biofilm. Subsequently, a strain lacking a blue light-sensing protein (YcgF) was constructed via genetic engineering, which substantially reduced the inhibitory effect of blue light signaling on biofilm. A high-efficiency biofilm-forming system, Magnet, was constructed, which enhanced bacterial aggregation and biofilm formation. Furthermore, l-threonine production was increased from 10.12 to 16.57 g/L during immobilized fermentation, and the fermentation period was shortened by 6 h. IMPORTANCE We confirmed the mechanism underlying the inhibitory effects of blue light signaling on E. coli biofilm formation and constructed a strain lacking a blue light-sensing protein; this mitigated the aforementioned effects of blue light signaling and ensured normal fermentation performance. Furthermore, this study elucidated that the blue light signaling-induced optogenetic system Magnet effectively regulates E. coli biofilm formation and contributes to l-threonine production. This study not only enriches the mechanism of blue light signaling to regulate E. coli biofilm formation but also provides a theoretical basis and feasibility reference for the application of optogenetics technology in biofilm-based immobilized fermentation systems.
Keywords: Escherichia coli; biofilm; blue light signaling; l-threonine; optogenetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Thieme L, Hartung A, Tramm K, Klinger-Strobel M, Jandt KD, Makarewicz O, Pletz MW. 2019. MBEC versus MBIC: the lack of differentiation between biofilm reducing and inhibitory effects as a current problem in biofilm methodology. Biol Proced Online 21:18. doi:10.1186/s12575-019-0106-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

