Platelet degranulation and bleeding phenotype in a large cohort of Von Willebrand disease patients
- PMID: 36165954
- PMCID: PMC9314899
- DOI: 10.1111/bjh.18145
Platelet degranulation and bleeding phenotype in a large cohort of Von Willebrand disease patients
Abstract
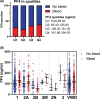
Von Willebrand disease (VWD) is a bleeding disorder caused by quantitative (type 1 or 3) or qualitative (type 2A/2B/2M/2N) defects of circulating von Willebrand factor (VWF). Circulating VWF levels not always fully explain bleeding phenotypes, suggesting a role for alternative factors, like platelets. Here, we investigated platelet factor 4 (PF4) in a large cohort of patients with VWD. PF4 levels were lower in type 2B and current bleeding phenotype was significantly associated with higher PF4 levels, particularly in type 1 VWD. Based on our findings we speculate that platelet degranulation and cargo release may play a role across VWD subtypes.
Keywords: VWD; VWF; bleeding disorders; platelet activation; platelet factor 4.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
F. W. G. Leebeek received research support from CSL Behring and Shire for performing the Willebrand in the Netherlands (WiN) study, and is consultant for uniQure, Biomarin, Novo Nordisk and Shire, of which the fees go to the institution. F. Atiq received the CSL Behring‐Heimburger Award 2018, and a travel grant from Sobi. I. van Moort received the CSL Behring‐Heimburger Award 2021. A. J. G. Jansen received speaker fees and travel cost payments from 3SBio, Amgen and Novartis, is on the international advisory board at Novartis and received research support from Sanofi, Argenx and CSL Behring. J. Eikenboom received research support from CSL Behring and he has been a teacher on educational activities of Roche. K. P. M. van Galen received unrestricted research support from CSL Behring and Bayer. J. G. van der Bom has received unrestricted research/educational funding for various projects from the following companies: Bayer Schering Pharma, Baxter, CSL Behring, Novo Nordisk, and Pfizer. In addition, she has been a consultant to Baxter and Pfizer, and she has been a teacher on educational activities of Bayer Schering Pharma. M. H. Cnossen has received unrestricted research/educational and travel funding from the following companies: Pfizer, Baxter, Bayer Schering Pharma, CSL Behring, Novo Nordisk and Novartis, and serves as a member on steering boards of Roche and Bayer of which fees go to the institution. K. Fijnvandraat is a member of the European Haemophilia Treatment and Standardization Board sponsored by Baxter, has received unrestricted research grants from CSL Behring and Bayer, and has given lectures at educational symposiums organized by Pfizer, Bayer and Baxter. K. Meijer received speaker fees from Alexion, Bayer and CSL Behring, fees for participation in trial steering committee for Bayer, consulting fees from Uniqure, and fees for participation in data monitoring and endpoint adjudication committee for Octapharma. S. Schols received travel grants from Bayer and Takeda and consultancy grants from Takeda and Novo Nordisk. None of the other authors has a conflict of interest to declare.
Figures

References
-
- Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. 2016;375(21):2067–80. - PubMed
-
- de Wee EM, Sanders YV, Mauser‐Bunschoten EP, van der Bom JG, Degenaar‐Dujardin ME, Eikenboom J, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683–92. - PubMed
-
- Karampini E, Bierings R, Voorberg J. Orchestration of primary hemostasis by platelet and endothelial lysosome‐related organelles. Arterioscler Thromb Vasc Biol. 2020;40(6):1441–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

