Pathophysiology of type 2 diabetes in sub-Saharan Africans
- PMID: 36166072
- PMCID: PMC9630207
- DOI: 10.1007/s00125-022-05795-2
Pathophysiology of type 2 diabetes in sub-Saharan Africans
Abstract
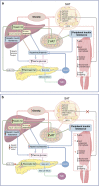
Sub-Saharan Africa (SSA) is the region with the highest projected rates of increase in type 2 diabetes (129% by 2045), which will exacerbate the already high prevalence of type 2 diabetes complications and comorbidities in SSA. In addition, SSA is grappling with poverty-related health problems and infectious diseases and is also undergoing the most rapid rates of urbanisation globally. These socioenvironmental and lifestyle factors may interact with genetic factors to alter the pathophysiological sequence leading to type 2 diabetes in sub-Saharan African populations. Indeed, current evidence from SSA and the diaspora suggests that the pathophysiology of type 2 diabetes in Black Africans is different from that in their European counterparts. Studies from the diaspora suggest that insulin clearance is the primary defect underlying the development of type 2 diabetes. We propose that, among Black Africans from SSA, hyperinsulinaemia due to a combination of both increased insulin secretion and reduced hepatic insulin clearance is the primary defect, which promotes obesity and insulin resistance, exacerbating the hyperinsulinaemia and eventually leading to beta cell failure and type 2 diabetes. Nonetheless, the current understanding of the pathogenesis of type 2 diabetes and the clinical guidelines for preventing and managing the disease are largely based on studies including participants of predominately White European ancestry. In this review, we summarise the existing knowledge base and data from the only non-pharmacological intervention that explores the pathophysiology of type 2 diabetes in SSA. We also highlight factors that may influence the pathogenesis of type 2 diabetes in SSA, such as social determinants, infectious diseases and genetic and epigenetic influences.
Keywords: Beta cell function; Epigenetics; Ethnicity; Genetics; Hyperinsulinaemia; Infectious diseases; Insulin resistance; Insulin sensitivity; Obesity; Review; Social determinants.
© 2022. The Author(s).
Figures



Comment in
-
Environmental exposures are important for type 2 diabetes pathophysiology in sub-Saharan African populations.Diabetologia. 2023 Apr;66(4):777-779. doi: 10.1007/s00125-022-05867-3. Epub 2023 Jan 19. Diabetologia. 2023. PMID: 36656321 No abstract available.
-
Environmental exposures are important for type 2 diabetes pathophysiology in sub-Saharan African populations. Reply to Christensen D, Hjort L, Mpagama S et al [letter].Diabetologia. 2023 Apr;66(4):780-782. doi: 10.1007/s00125-022-05859-3. Epub 2023 Jan 24. Diabetologia. 2023. PMID: 36692507 No abstract available.
-
A large portion of diabetes cases in sub-Saharan African populations with HIV represent drug-induced diabetes.Diabetologia. 2023 Jun;66(6):1162-1164. doi: 10.1007/s00125-023-05904-9. Epub 2023 Mar 23. Diabetologia. 2023. PMID: 36949292 No abstract available.
References
-
- Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. 10.1016/j.diabres.2019.107843 - PubMed
-
- Goedecke JH, Olsson T (2020) Pathogenesis of type 2 diabetes risk in black Africans: a South African perspective. J Intern Med 288(3):284–294. 10.1111/joim.13083 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

