Immobilization-Free Binding and Affinity Characterization of Higher Order Bispecific Antibody Complexes Using Size-Based Microfluidics
- PMID: 36166291
- PMCID: PMC9558742
- DOI: 10.1021/acs.analchem.2c02705
Immobilization-Free Binding and Affinity Characterization of Higher Order Bispecific Antibody Complexes Using Size-Based Microfluidics
Abstract
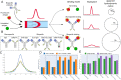
Simultaneous targeting of different antigens by bispecific antibodies (bsAbs) is permitting synergistic binding functionalities with high therapeutic potential, but is also rendering their analysis challenging. We introduce flow-induced dispersion analysis (FIDA) for the in-depth characterization of bsAbs with diverse molecular architectures and valencies under near-native conditions without potentially obstructive surface immobilization. Individual equilibrium dissociation constants are determined in solution, even in higher-order complexes with both antigens involved, hereby allowing the analysis of binding cooperativity and elucidation of a potential interference between the interactions. We further illustrate bispecific binding functionality as incremental increases in complex sizes when the bsAbs are exposed to one or two antigens. The possibility for comprehensive binding analysis with low material consumption and high matrix tolerability irrespective of molecular format and with little optimization renders FIDA a versatile tool for format selection and characterization of complex bi/multispecific protein therapeutics throughout the drug development and biomanufacturing pipeline.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Huang S.; Li C.; Armstrong E. A.; Peet C. R.; Saker J.; Amler L. C.; Sliwkowski M. X.; Harari P. M. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer research 2013, 73 (2), 824–833. 10.1158/0008-5472.CAN-12-1611. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

