Interleukin-37 protects against acinar cell pyroptosis in acute pancreatitis
- PMID: 36166295
- PMCID: PMC9675483
- DOI: 10.1172/jci.insight.161244
Interleukin-37 protects against acinar cell pyroptosis in acute pancreatitis
Abstract
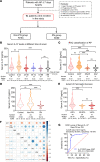
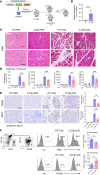
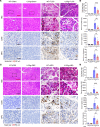
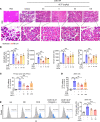
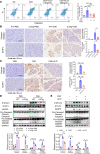
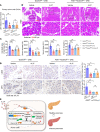
Acute pancreatitis (AP) is a local and/or systemic inflammatory disease that starts with acinar cell injury and necrosis; it has no effective medical treatment and thus remains a life-threatening condition. Interleukin-37 (IL-37), a natural immunomodulator, has demonstrated an antiinflammatory effect; however, the role of IL-37 in AP remains unknown. The serum IL-37 levels of 39 healthy controls and 94 patients with AP were measured. Cholecystokinin was applied to induce pancreatic acinar cell injury in vitro. Classical experimental AP models, such as caerulein, l-arginine, and taurolithocholic acid 3-sulfate disodium salt, were included in the in vivo study. A transgenic mouse model with the IL-37 gene and administration of recombinant IL-37 were used to further investigate the function of IL-37 in AP. Pancreas-specific gasdermin D-knockout (GSDMD-knockout) mice were used to explore the protective mechanism of IL-37. Our results showed that serum IL-37 levels in humans were negatively correlated with the severity of AP. Furthermore, IL-37-transgenic mice and supplementation with recombinant IL-37 could both protect against AP. Mechanistically, IL-37 was able to suppress pyroptosis of injured acinar cells, and specific depletion of GSDMD in the pancreas counteracted the protective effect of IL-37. Our study demonstrates that IL-37 protects against acinar cell pyroptosis in AP.
Keywords: Cytokines; Gastroenterology; Inflammation; Pharmacology.
Figures
References
-
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

