DIO3 protects against thyrotoxicosis-derived cranio-encephalic and cardiac congenital abnormalities
- PMID: 36166296
- PMCID: PMC9675556
- DOI: 10.1172/jci.insight.161214
DIO3 protects against thyrotoxicosis-derived cranio-encephalic and cardiac congenital abnormalities
Abstract
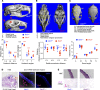
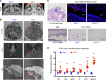
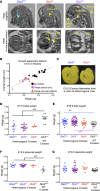
Maternal hyperthyroidism is associated with an increased incidence of congenital abnormalities at birth, but it is not clear which of these defects arise from a transient developmental excess of thyroid hormone and which depend on pregnancy stage, antithyroid drug choice, or unwanted subsequent fetal hypothyroidism. To address this issue, we studied a mouse model of comprehensive developmental thyrotoxicosis secondary to a lack of type 3 deiodinase (DIO3). Dio3-/- mice exhibited reduced neonatal viability on most genetic backgrounds and perinatal lethality on a C57BL/6 background. Dio3-/- mice exhibited severe growth retardation during the neonatal period and cartilage loss. Mice surviving after birth manifested brain and cranial dysmorphisms, severe hydrocephalus, choanal atresia, and cleft palate. These abnormalities were noticeable in C57BL/6J Dio3-/- mice at fetal stages, in addition to a thyrotoxic heart with septal defects and thin ventricular walls. Our findings stress the protecting role of DIO3 during development and support the hypothesis that human congenital abnormalities associated with hyperthyroidism during pregnancy are caused by transient thyrotoxicosis before clinical intervention. Our results also suggest thyroid hormone involvement in the etiology of idiopathic pathologies including cleft palate, choanal atresia, Chiari malformations, Kaschin-Beck disease, and Temple and other cranio-encephalic and heart syndromes.
Keywords: Development; Embryonic development; Endocrinology; Mouse models.
Conflict of interest statement
Figures





Similar articles
-
Transgenerational epigenetic self-memory of Dio3 dosage is associated with Meg3 methylation and altered growth trajectories and neonatal hormones.Epigenetics. 2024 Dec;19(1):2376948. doi: 10.1080/15592294.2024.2376948. Epub 2024 Jul 11. Epigenetics. 2024. PMID: 38991122 Free PMC article.
-
Decreased anxiety- and depression-like behaviors and hyperactivity in a type 3 deiodinase-deficient mouse showing brain thyrotoxicosis and peripheral hypothyroidism.Psychoneuroendocrinology. 2016 Dec;74:46-56. doi: 10.1016/j.psyneuen.2016.08.021. Epub 2016 Aug 24. Psychoneuroendocrinology. 2016. PMID: 27580013 Free PMC article.
-
Adult onset of type 3 deiodinase deficiency in mice alters brain gene expression and increases locomotor activity.Psychoneuroendocrinology. 2019 Dec;110:104439. doi: 10.1016/j.psyneuen.2019.104439. Epub 2019 Sep 18. Psychoneuroendocrinology. 2019. PMID: 31561084 Free PMC article.
-
The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function.Int J Mol Sci. 2018 Jun 19;19(6):1804. doi: 10.3390/ijms19061804. Int J Mol Sci. 2018. PMID: 29921775 Free PMC article. Review.
-
Foetal and neonatal thyroid disorders.Minerva Pediatr. 2002 Oct;54(5):383-400. Minerva Pediatr. 2002. PMID: 12244277 Review. English, Italian.
Cited by
-
Editorial for Special Issue: "MicroRNA in Cardiac Health and Disease".Int J Mol Sci. 2022 Dec 8;23(24):15567. doi: 10.3390/ijms232415567. Int J Mol Sci. 2022. PMID: 36555208 Free PMC article.
-
Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain.Elife. 2023 May 19;12:e82683. doi: 10.7554/eLife.82683. Elife. 2023. PMID: 37204837 Free PMC article.
-
Year in Thyroidology: Basic Science.Thyroid. 2023 Jan;33(1):16-20. doi: 10.1089/thy.2022.0633. Epub 2022 Dec 28. Thyroid. 2023. PMID: 36465054 Free PMC article. No abstract available.
-
Congenital hydrocephalus: a review of recent advances in genetic etiology and molecular mechanisms.Mil Med Res. 2024 Aug 12;11(1):54. doi: 10.1186/s40779-024-00560-5. Mil Med Res. 2024. PMID: 39135208 Free PMC article. Review.
-
Epigenetic developmental programming and intergenerational effects of thyroid hormones.Vitam Horm. 2023;122:23-49. doi: 10.1016/bs.vh.2023.01.003. Epub 2023 Feb 9. Vitam Horm. 2023. PMID: 36863795 Free PMC article. Review.
References
-
- Legrand J. Effects of thyroid hormones on central nervous system development. In: Yanai J, ed. Neurobehavioral Teratology. Elsevier; 1984:331–363.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

