Tick feeding modulates the human skin immune landscape to facilitate tick-borne pathogen transmission
- PMID: 36166299
- PMCID: PMC9621130
- DOI: 10.1172/JCI161188
Tick feeding modulates the human skin immune landscape to facilitate tick-borne pathogen transmission
Abstract
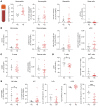
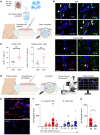
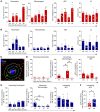
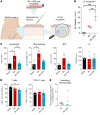
During cutaneous tick attachment, the feeding cavity becomes a site of transmission for tick salivary compounds and tick-borne pathogens. However, the immunological consequences of tick feeding for human skin remain unclear. Here, we assessed human skin and blood samples upon tick bite and developed a human skin explant model mimicking Ixodes ricinus bites and tick-borne pathogen infection. Following tick attachment, we observed rapidly occurring patterns of immunomodulation, including increases in neutrophils and cutaneous B and T cells. T cells upregulated tissue residency markers, while lymphocytic cytokine production was impaired. In early stages of Borrelia burgdorferi model infections, we detected strain-specific immune responses and close spatial relationships between macrophages and spirochetes. Preincubation of spirochetes with tick salivary gland extracts hampered accumulation of immune cells and increased spirochete loads. Collectively, we showed that tick feeding exerts profound changes on the skin immune network that interfere with the primary response against tick-borne pathogens.
Keywords: Adaptive immunity; Immunology; Skin.
Conflict of interest statement
Figures



References
-
- European Centre for Disease Prevention and Control and European Food Safety Authority. Tick maps. https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-d... Updated 13 April 12, 2022. Accessed August 25, 2022.

