Cost-Effectiveness of Colorectal Cancer Surveillance in Hodgkin Lymphoma Survivors Treated with Procarbazine and/or Infradiaphragmatic Radiotherapy
- PMID: 36166472
- PMCID: PMC9720424
- DOI: 10.1158/1055-9965.EPI-22-0019
Cost-Effectiveness of Colorectal Cancer Surveillance in Hodgkin Lymphoma Survivors Treated with Procarbazine and/or Infradiaphragmatic Radiotherapy
Abstract
Background: Hodgkin lymphoma survivors treated with infradiaphragmatic radiotherapy (IRT) and/or procarbazine have an increased risk of developing colorectal cancer. We investigated the cost-effectiveness of colorectal cancer surveillance in Dutch Hodgkin lymphoma survivors to determine the optimal surveillance strategy for different Hodgkin lymphoma subgroups.
Methods: The Microsimulation Screening Analysis-Colon model was adjusted to reflect colorectal cancer and other-cause mortality risk in Hodgkin lymphoma survivors. Ninety colorectal cancer surveillance strategies were evaluated varying in starting and stopping age, interval, and modality [colonoscopy, fecal immunochemical test (FIT, OC-Sensor; cutoffs: 10/20/47 μg Hb/g feces), and multi-target stool DNA test (Cologuard)]. Analyses were also stratified per primary treatment (IRT and procarbazine or procarbazine without IRT). Colorectal cancer deaths averted (compared with no surveillance) and incremental cost-effectiveness ratios (ICER) were primary outcomes. The optimal surveillance strategy was identified assuming a willingness-to-pay threshold of €20,000 per life-years gained (LYG).
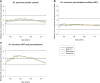
Results: Overall, the optimal surveillance strategy was annual FIT (47 μg) from age 45 to 70 years, which might avert 70% of colorectal cancer deaths in Hodgkin lymphoma survivors (compared with no surveillance; ICER:€18,000/LYG). The optimal surveillance strategy in Hodgkin lymphoma survivors treated with procarbazine without IRT was biennial FIT (47 μg) from age 45 to 70 years (colorectal cancer mortality averted 56%; ICER:€15,000/LYG), and when treated with IRT and procarbazine, annual FIT (47 μg) surveillance from age 40 to 70 was most cost-effective (colorectal cancer mortality averted 75%; ICER:€13,000/LYG).
Conclusions: Colorectal cancer surveillance in Hodgkin lymphoma survivors is cost-effective and should commence earlier than screening occurs in population screening programs. For all subgroups, FIT surveillance was the most cost-effective strategy.
Impact: Colorectal cancer surveillance should be implemented in Hodgkin lymphoma survivors.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- 1055-9965. doi: 10.1158/1055-9965.EPI-31-12-HI doi: 10.1158/1055-9965.EPI-31-12-HI
References
-
- Schaapveld M, Aleman BM, van Eggermond AM, Janus CP, Krol AD, van der Maazen RW, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med 2015;373:2499–511. - PubMed
-
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, et al. Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol 2011;29:4096–104. - PubMed
-
- Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 2007;25:1489–97. - PubMed
-
- Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomized controlled trial. Lancet Oncol 2012;13:55–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

