Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells
- PMID: 36167294
- PMCID: PMC10334248
- DOI: 10.1016/j.jare.2022.09.007
Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells
Abstract
Introduction: Inherent or acquired resistance to paclitaxel (PTX) is a pivotal challenge for chemotherapy treatment of multidrug-resistant (MDR) breast cancer. Although various targeted drug-delivery systems, including nanoparticles and liposomes, are effective for MDR cancer treatment, their efficacy is restricted by immunosuppressive tumor microenvironment (TME).
Methods: Ginsenosides Rg3 was used to formulate unique Rg3-based liposomes loaded with PTX to establish Rg3-PTX-LPs, which were prepared by the thin-film hydration method. The stability of the Rg3-PTX-LPs was evaluated by particle size analysis through dynamic light scattering. The active targeting effect of Rg3-based liposomes was examined in an MCF-7/T xenograft model by an in a vivo imaging system. To evaluate the antitumor activity and mechanism of Rg3-PTX-LP, MTT, apoptosis assays, TAM regulation, and TME remodeling were performed in MCF-7/T cells in vitro and in vivo.
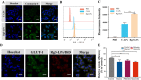
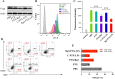
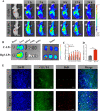
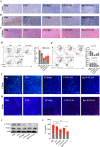
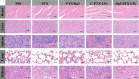
Results: Rg3-PTX-LPs could specifically distribute to MCF7/T cancer cells and TME simultaneously, mainly through the recognition of GLUT-1. The drug resistance reversing capability and in vivo antitumor effect of Rg3-PTX-LPs were significantly improved compared with conventional cholesterol liposomes. The TME remodeling mechanisms of Rg3-PTX-LPs included inhibiting IL-6/STAT3/p-STAT3 pathway activation to repolarize protumor M2 macrophages to antitumor M1 phenotype, suppressing myeloid-derived suppressor cells (MDSCs), decreasing tumor-associated fibroblasts (TAFs) and collagen fibers in TME, and promoting apoptosis of tumor cells. Hence, through the dual effects of targeting tumor cells and TME remodeling, Rg3-PTX-LPs achieved a high tumor inhibition rate of 90.3%.
Conclusion: Our multifunctional Rg3-based liposome developed in the present study offered a promising strategy for rescuing the drug resistance tumor treatment.
Keywords: Ginsenoside Rg3; Liposomes; MCF-7/T tumor; Multidrug resistance; Paclitaxel; Tumor microenvironment remodeling.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Rutqvist L.E., Cedermark B., Glas U., Johansson H., Rotstein S., Skoog L., et al. Radiotherapy, chemotherapy, and tamoxifen as adjuncts to surgery in early breast cancer: a summary of three randomized trials. Int J Radiat Oncol Biol Phys. 1989;16(3):629–639. - PubMed
-
- Huang Y., Li Y. Drug Delivery and Reversal of MDR. Mol Pharm. 2014;11(8):2493–2494. - PubMed
-
- Ling V. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol. 1997;40(7):S3–S8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

