Bacterial AB toxins and host-microbe interactions
- PMID: 36167443
- PMCID: PMC12035773
- DOI: 10.1016/bs.ampbs.2022.06.002
Bacterial AB toxins and host-microbe interactions
Abstract
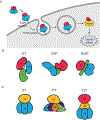
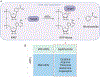
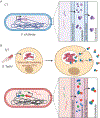
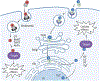
AB toxins are protein virulence factors secreted by many bacterial pathogens, contributing to the pathogenicity of the cognate bacteria. AB toxins consist of two functionally distinct components: the enzymatic "A" component for pathogenicity and the receptor-binding "B" component for toxin delivery. Consistently, unlike other virulence factors such as effectors, AB toxins do not require additional systems to deliver them to the target host cells. Target host cells are located in the infection site and/or located distantly from infected host cells. The first part of this review discusses the structural and functional features of single-peptide and multiprotein AB toxins in the context of host-microbe interactions, using several well-characterized examples. The second part of this review discusses toxin neutralization strategies, as well as applications of AB toxins relevant to developing intervention strategies against diseases.
Keywords: AB toxin; Adjuvant; Antibody; Bacteria; Host and microbe interaction; Pathogen; Pathogenicity; Structure and function; Toxin neutralization; Toxin synthesis, secretion, and delivery; Vaccine; Virulence.
Copyright © 2022 Elsevier Ltd All rights reserved.
Figures
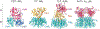
Similar articles
-
Delivery, structure, and function of bacterial genotoxins.Virulence. 2022 Dec;13(1):1199-1215. doi: 10.1080/21505594.2022.2097417. Virulence. 2022. PMID: 35795909 Free PMC article. Review.
-
Coordinated delivery and function of bacterial MARTX toxin effectors.Mol Microbiol. 2018 Jan;107(2):133-141. doi: 10.1111/mmi.13875. Epub 2017 Dec 5. Mol Microbiol. 2018. PMID: 29114985 Free PMC article. Review.
-
Toxins from bacteria.EXS. 2010;100:1-29. doi: 10.1007/978-3-7643-8338-1_1. EXS. 2010. PMID: 20358680 Free PMC article. Review.
-
Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles.Front Cell Infect Microbiol. 2020 Mar 6;10:91. doi: 10.3389/fcimb.2020.00091. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32211344 Free PMC article. Review.
-
Molecular weaponry: diverse effectors delivered by the Type VI secretion system.Cell Microbiol. 2015 Dec;17(12):1742-51. doi: 10.1111/cmi.12532. Epub 2015 Nov 3. Cell Microbiol. 2015. PMID: 26432982 Free PMC article. Review.
Cited by
-
Malnutrition and maternal vaccination against typhoid toxin.PLoS Pathog. 2022 Aug 12;18(8):e1010731. doi: 10.1371/journal.ppat.1010731. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35960787 Free PMC article.
-
Molecular basis of the hepatobiliary tropism of typhoid toxin promoting Salmonella pathogenicity.Sci Adv. 2025 Jun 6;11(23):eadt2040. doi: 10.1126/sciadv.adt2040. Epub 2025 Jun 6. Sci Adv. 2025. PMID: 40479051 Free PMC article.
-
Overview of Bacterial Protein Toxins from Pathogenic Bacteria: Mode of Action and Insights into Evolution.Toxins (Basel). 2024 Apr 8;16(4):182. doi: 10.3390/toxins16040182. Toxins (Basel). 2024. PMID: 38668607 Free PMC article. Review.
References
-
- Aoki KR, & Guyer B (2001). Botulinum toxin type a and other botulinum toxin serotypes: A comparative review of biochemical and pharmacological actions. European Journal of Neurology, 8(Suppl 5), 21–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

