Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors
- PMID: 36167467
- PMCID: PMC9516314
- DOI: 10.1136/jitc-2021-004450
Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors
Abstract
Background: Pediatric brain tumors are the leading cause of cancer death in children with an urgent need for innovative therapies. Glypican 2 (GPC2) is a cell surface oncoprotein expressed in neuroblastoma for which targeted immunotherapies have been developed. This work aimed to characterize GPC2 expression in pediatric brain tumors and develop an mRNA CAR T cell approach against this target.
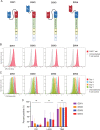
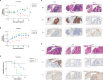
Methods: We investigated GPC2 expression across a cohort of primary pediatric brain tumor samples and cell lines using RNA sequencing, immunohistochemistry, and flow cytometry. To target GPC2 in the brain with adoptive cellular therapies and mitigate potential inflammatory neurotoxicity, we used optimized mRNA to create transient chimeric antigen receptor (CAR) T cells. We developed four mRNA CAR T cell constructs using the highly GPC2-specific fully human D3 single chain variable fragment for preclinical testing.
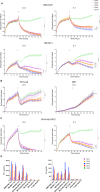
Results: We identified high GPC2 expression across multiple pediatric brain tumor types including medulloblastomas, embryonal tumors with multilayered rosettes, other central nervous system embryonal tumors, as well as definable subsets of highly malignant gliomas. We next validated and prioritized CAR configurations using in vitro cytotoxicity assays with GPC2-expressing neuroblastoma cells, where the light-to-heavy single chain variable fragment configurations proved to be superior. We expanded the testing of the two most potent GPC2-directed CAR constructs to GPC2-expressing medulloblastoma and high-grade glioma cell lines, showing significant GPC2-specific cell death in multiple models. Finally, biweekly locoregional delivery of 2-4 million GPC2-directed mRNA CAR T cells induced significant tumor regression in an orthotopic medulloblastoma model and significantly prolonged survival in an aggressive orthotopic thalamic diffuse midline glioma xenograft model. No GPC2-directed CAR T cell related neurologic or systemic toxicity was observed.
Conclusion: Taken together, these data show that GPC2 is a highly differentially expressed cell surface protein on multiple malignant pediatric brain tumors that can be targeted safely with local delivery of mRNA CAR T cells, laying the framework for the clinical translation of GPC2-directed immunotherapies for pediatric brain tumors.
Keywords: Brain Neoplasms; Cell Engineering; Central Nervous System Neoplasms; Immunotherapy; Pediatrics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TS is currently employed by Spark Therapeutics. KK is currently employed by BioNTech and is an inventor on a patent related to use of nucleoside-modified mRNA. DMB is currently employed by Tmunity Therapeutics. JBF, DMB, JMM, and KRB hold patents for the discovery and development of immunotherapies for cancer, including patents related to glypican 2 (GPC2)-directed immunotherapies. KRB and JMM receive research funding from Tmunity for research on GPC2-directed immunotherapies and JBF, DMB, JMM, and KRB receive royalties from Tmunity for licensing of GPC2-related intellectual property. JMM is a founder of both Tantigen Bio and Hula Therapeutics, focused on cellular therapies for childhood cancers, but neither are working on GPC2-directed therapeutics. All other authors have nothing to disclose.
Figures



References
-
- Gardner R, Finney O, Smithers H, et al. . CD19CAR T Cell Products of Defined CD4:CD8 Composition and Transgene Expression Show Prolonged Persistence and Durable MRD-Negative Remission in Pediatric and Young Adult B-Cell ALL. Blood 2016;128:219. 10.1182/blood.V128.22.219.219 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
