Effectiveness of a digital medication event reminder and monitor device for patients with tuberculosis (SELFTB): a multicenter randomized controlled trial
- PMID: 36167528
- PMCID: PMC9514884
- DOI: 10.1186/s12916-022-02521-y
Effectiveness of a digital medication event reminder and monitor device for patients with tuberculosis (SELFTB): a multicenter randomized controlled trial
Abstract
Background: Tuberculosis remains the leading cause of death from a single infectious disease worldwide. Trials evaluating digital adherence technologies for tuberculosis in low- and middle-income countries are urgently needed. We aimed to assess whether a digital medication event reminder and monitor (MERM) device-observed self-administered therapy improves adherence and treatment outcomes in patients with tuberculosis compared with the standard in-person directly observed therapy (DOT).
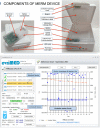
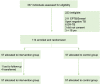
Methods: We did a two-arm, attention-controlled, effectiveness-implementation type 2 hybrid, randomized controlled trial in ten healthcare facilities in Addis Ababa, Ethiopia. We included adults with new or previously treated, bacteriologically confirmed, drug-sensitive pulmonary tuberculosis who were eligible to start anti-tuberculosis therapy. Participants were randomly assigned (1:1) to receive a 15-day tuberculosis medication supply in the evriMED500® MERM device to self-administer and return every 15 days (intervention arm) or visit the healthcare facilities each day to swallow their daily dose with DOT by healthcare providers (control arm). Both arms were followed throughout the standard two-month intensive treatment phase (2RHZE). For control participants, some provider-approved take-home doses might be allowed for extenuating circumstances in real-world practice. Data were collected on patient information (demographic, socioeconomic, behavioral, social, and clinical information), medication adherence measures (MERM vs. DOT records, IsoScreenTM urine colorimetric isoniazid test, and adherence self-report), and clinical measures (pre-post treatment sputum Xpert MTB/RIF assay or microscopy, and adverse treatment outcomes). The intention-to-treat (ITT) primary endpoints were (1) individual-level percentage adherence over the two-month intensive phase measured by adherence records compiled from MERM device vs. DOT records that also considered all take-home doses as having been ingested and (2) sputum smear conversion following the standard two-month intensive phase treatment. Secondary endpoints were (1) individual-level percentage adherence over the two-month intensive phase measured by adherence records compiled from the MERM device vs. DOT records that considered all take-home doses as not ingested, (2) negative IsoScreen urine isoniazid test, (3) adverse treatment outcome (having at least one of the three events: treatment not completed; death; or loss to follow-up), and (4) self-reported adherence. The MERM device has an electronic module and a medication container that records adherence, stores medication, emits audible and visual on-board alarms to remind patients to take their medications on time and refill, and enables providers to download the data and monitor adherence.
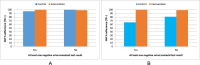
Results: Participants were enrolled into the study between 02 June 2020 and 15 June 2021, with the last participant completing follow-up on 15 August 2021. A total of 337 patients were screened for eligibility, of whom 114 were randomly assigned and included in the final analysis [57 control and 57 intervention participants]. Participants were 64.9% male, 15% with HIV, 10.5% retreatment, and 5.3% homeless. Adherence to TB medication was comparable between the intervention arm [geometric mean percentage (GM%) 99.01%, geometric standard deviation (GSD) 1.02] and the control arm [GM% 98.97%, GSD 1.04] and was within the prespecified margin for non-inferiority [mean ratio (MR) 1.00 (95% CI 0.99-1.01); p = 0.954]. The intervention arm was significantly superior to the control arm in the secondary analysis that considered all take-home doses in the control were not ingested [control GM% 77.71 (GSD 1.57), MR 1.27 (95% CI 1.33-1.43)]. Urine isoniazid testing was done on 443 (97%) samples from 114 participants; 13 participants had at least one negative result; a negative test was significantly more common among the control group compared with the intervention group [11/57 (19.3%) vs 2/57 (3.5%); p = 0.008]. There was no significant difference between the control and intervention arms for smear conversion [55 (98.2%) vs 52 (100%); p>0.999], adverse treatment outcomes [0 vs 1 (1.9%); p = 0.48], and self-report non-adherence [5 (8.9%) vs 1 (1.9%); p = 0.21].
Conclusions: In this randomized trial of patients with drug-sensitive pulmonary tuberculosis, medication adherence among participants assigned to MERM-observed self-administered therapy was non-inferior and superior by some measures when compared with the standard in-person DOT. Further research is needed to understand whether adherence in the intervention is primarily driven by allowing self-administered therapy which reduced challenges of repeated clinic visits or by the adherence support provided by the MERM system. To avoid contributing to patient barriers with DOT, tuberculosis medical programs should consider alternatives such as medication event monitors.
Trial registration: ClinicalTrials.gov, NCT04216420.
Keywords: Adherence; Clinical trial; Digital health; Directly observed therapy (DOT); Ethiopia; Medication event reminder monitor (MERM); Self-administered therapy; Treatment; Tuberculosis; Urine isoniazid testing.
© 2022. The Author(s).
Conflict of interest statement
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV. All other authors report no potential conflicts.
Figures

References
-
- World Health Organization (WHO). Global tuberculosis report 2021. Geneva, Switzerland, WHO 2021. https://www.who.int/publications/i/item/9789240037021. (Accessed 18 October 2021).
-
- Manyazewal T, Woldeamanuel Y, Holland DP, Fekadu A, Blumberg HM, Marconi VC. Electronic pillbox-enabled self-administered therapy versus standard directly observed therapy for tuberculosis medication adherence and treatment outcomes in Ethiopia (SELFTB): protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):383. doi: 10.1186/s13063-020-04324-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

